��Ŀ����
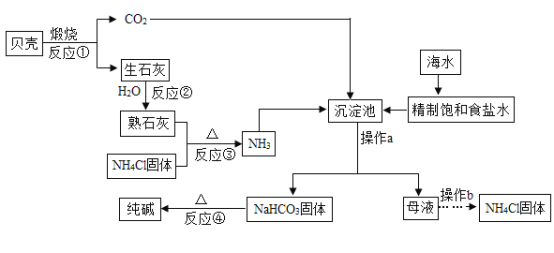
����Ŀ���ڹ���������Һ�ķֽⷴӦ�У�ij��ѧ��ȤС�鷢�֣����˶������̣�����ͭ��ҺҲ�ܶ�H2O2�ķֽ�������á��Դˣ����ǽ������й�̽����
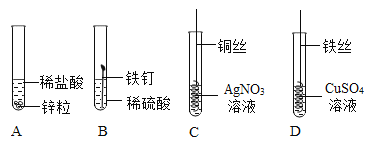
��������⣩CuSO4��Һ�ǻ�����������һ�ֳɷ����˴������أ�
���������ϣ���ϡ��������Ҫ����H2O��H+��SO42��������
��CuSO4��Һ����Ҫ����H2O��Cu2+��SO42��������
���������룩����������Ϣ��ͬѧ�������������������룺
��SO42���ֽ�H2O2 ��Cu2+���ֽ�H2O2 ��H2O���ֽ�H2O2
��1�����У����Բ���������_______������ţ���������_______��
��ʵ����֤��
��2��Ϊ����֤�Լ��IJ��룬ͬѧ���������������ʵ�飨��ȡH2O2��Һ��Ũ����ͬ����
���� | ���� | ���� |
ȡ5 mL H2O2��Һ���Թ��У�����������ϡ���ᣬ��������ǵ�ľ�� | _______ | SO42��H2O2�ķֽⲻ������� |
_______ | �����������ݣ������ǵ�ľ����ȼ | _______ |
��ʵ����չ��
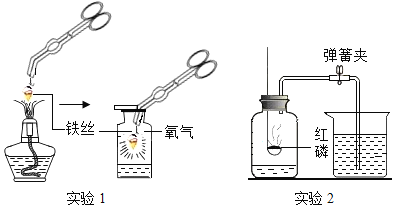
ʵ��һ����ͼ��װ��̽��Ӱ��H2O2�ֽ����ʵ����أ��õ�ͼ�ҡ�ͼ����ʾ���ߣ��ڵ��������£��������������װ����ѹǿ�����ȣ���Ӧ���Ⱥ��Բ��ƣ���
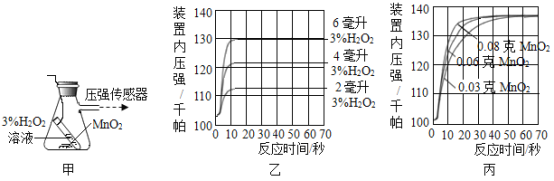
��3��д��ͼ���з�Ӧ�Ļ�ѧ����ʽ_______��
��4��ͼ���ǡ�0.1 g MnO2�벻ͬ�����3% H2O2��Һ��ϡ���ʵ��������ͼ�п��Կ���_______��
��5��ͼ�����á�8 mLŨ��Ϊ3%��H2O2��Һ�벻ͬ������MnO2��ϡ�ʱ�����õ������ߣ�ͨ�������߿��Եõ��Ľ�����_______��
ʵ�������ͼ��װ��̽��������Ũ�ȶ�ȼ�յ�Ӱ�졣��ȼ���������������Ƥ����������Ϩ����ι��е�ˮȫ������ƿ�У������еİ���ȼ�ա�
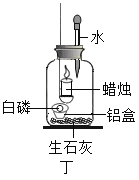
��6������ȼ�յ���Ҫ������_______��
��7���ɡ�����Ϩ�𣬰���ȼ�ա��ɵó��Ľ�����_______��
��8������ˮ�����ȼ�յ�ԭ����_______��
���𰸡��� H2O2��Һ�оͺ���H2O������δ�ӿ�������ݵ����� ���������ݣ�������ľ��δ��ȼ ȡ5 mL H2O2��Һ���Թ��У���������CuSO4��Һ�����������ľ�� Cu2+��H2O2�ķֽ�������ã���������ȷ�� 2H2O2  2H2O + O2����������дΪ��MnO2���� ������������Խ�࣬��������Խ�ࣨ�����������𰸣� MnO2������Խ��������������Խ�죨�����������𰸣� �������̣��ų����� ����ȼ����������Ũ�ȱȰ���ȼ����������Ũ��Ҫ�ߣ�������Ϩ�����ƿ������������ ��ʯ����ˮ��Ӧ���ȣ�ʹ�¶ȴﵽ�˰����Ż��
2H2O + O2����������дΪ��MnO2���� ������������Խ�࣬��������Խ�ࣨ�����������𰸣� MnO2������Խ��������������Խ�죨�����������𰸣� �������̣��ų����� ����ȼ����������Ũ�ȱȰ���ȼ����������Ũ��Ҫ�ߣ�������Ϩ�����ƿ������������ ��ʯ����ˮ��Ӧ���ȣ�ʹ�¶ȴﵽ�˰����Ż��
��������
��1��������ܵ�ԭ����H2O2�б�������ˮ����û�мӿ�������ݣ�
��2������ϡ�����Ŀ�����ṩ�����ӣ����ľ��û��ȼ��˵������SO42��Ĵ����ã����ų���SO42��H2O�ĸ��ź���Ȼ��Cu2+�����ã�ͨ��ģ��ʵ��һ�ķ�����ƿ���ѡ�������������CuSO4��Һ���ܹ��ṩͭ���ӣ���һ����֤��Cu2+��Ĵ����ã�
��3��װ�ü��й��������ڶ������̵Ĵ������·�Ӧ����ˮ��������������Ӧ�Ļ�ѧ����ʽΪ��2H2O2  2H2O + O2����
2H2O + O2����
��4��ͼ1������0.1gMnO2�벻ͬ�����3%H2O2��Һ�������ʵ��������ͼ�п��Կ�������������ҺԽ�࣬��������Խ�ࣻ
��5��ͼ��������8mLŨ��Ϊ3%��H2O2��Һ�벻ͬ������MnO2�����ʱ�����õ������ߣ�ͨ�������߿�֪���ڹ���������Һ��������Ũ����ͬ�������£��������̵�����Խ����������������Խ�죻
��6������ȼ�գ��ų�������ͬʱ�����˰��̣�
��7������Ϩ�������Ȼ�ܹ��ڼ���ƿ��ȼ�գ�˵������Ϩ�����ƿ����Ȼ����������
��8���ڸ�ʵ���У���ʯ�ҵ���Ҫ�����ǣ���ʯ����ˮ��Ӧ�ų��������ȣ��¶ȴﵽ�˰����Ż�㣬��ȼ���ס�

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д�����Ŀ��̽��ʵ�鷢�ֹ���
��1��̽��Ӱ�������ܽ��Ե�����.
ʵ��һ | ʵ��� |
|
|
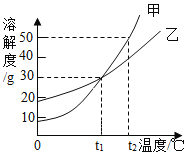
ʵ��һĿ����̽��__________��������ܽ��Ե�Ӱ��; ʵ����۲쵽��ʵ������Ϊ�Ȼ����ܽ���ˮ�������ܽ��ھƾ��С���ʵ���Ŀ����̽��__________���Ȼ����ܽ��Ե�Ӱ�죮
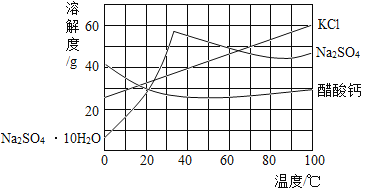
��2�������ܽ��������Ϣ�ش���������:�ס��ҡ����������ʣ��������ᾧˮ������ͼ��ʾ��

��20��ʱ���ס��ҡ������������ܽ���ɴ�С��˳��Ϊ__________��
����__________ʱ�����������ʵ��ܽ����ȡ������л��������ף��ɽ�����Һ__________�ᾧ���� �ȹ������ᴿ����
��50��ʱ�����������ļס��ҡ����������ʵı�����Һͬʱ������ 10����������Һ����������������С����__________��
����Ŀ���Ķ�������ն��ġ�
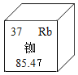
��ʯ����Ϊ������ͭ�Ļ��ķ���ء���ͭ�ųơ����𡱣���Ϊ���ɫ����Ҫ��ͭ������Ǧ�ĺϽ�����ͭ�������ɫ�� ������ͭ�������ִ�����������ͭ��������ʴ�ɷֿ��������Ƕ���ͭ����������о��ͱ�����
�о���Ա����X���������Ƕ��ҹ���������ijĹ�س�������ͭ�����������������ijɷֽ����˷����������������ķֲ�Ƶ����ͼ��
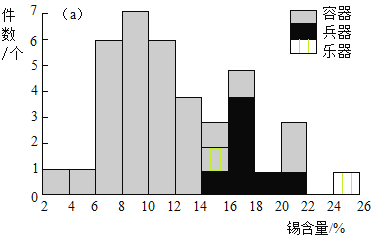
�о���Ա����X����������������ԡ����֮������ĸ�춦�����㲿λ����ʴ��Ʒ���з�����������£�
��ʴ�ɷ� ��Ʒ��� | Cu2Cl(OH)3 | Cu2O | Cu2CO3(OH)2 | SnO2 | ���� |
1 | 98% | 2% | 0 | 0 | 0 |
2 | 5% | 95% | 0 | 0 | 0 |
3 | 29% | 34% | 12% | 0 | 25% |
4 | 61% | 0 | 4% | 9% | 26% |
�����ɷ��У���ʽ�Ȼ�ͭ[Cu2Cl(OH)3]����ͭ�����Σ����������һ��մȾ���������ʣ��ڻ���ʪ�����˵������£��ͻ���������һ����Ⱦ�����ӣ�����������á����ף�ֱ�������߽⡣
�����������ݻش��������⡣
��1����ͭ������_________��
A �������ϡ� B �ϳɲ���
��2�����������ķֲ�Ƶ��ͼ�ƶϣ�ij����ͭ������������������_____
A 4% B 18%
��3����ĸ�춦�������ϵ���ʴ�ɷ��У�Cu2O����__________��
A ����� B ��
��4����ʽ�Ȼ�ͭ[Cu2Cl(OH)3]�������뻷���е�������ˮ��_________������ء�
A �Ȼ��� B ������̼
��5������Ա���������ͭ�����һ������������____________��