��Ŀ����
����Ŀ����֪�������˶�������������Ϧһ����ʵ�飬��������һ����Ҳ�̵����������������ÿ�ѧ����ʶ����ķ�ʽ��ʶ����������
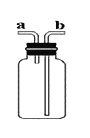
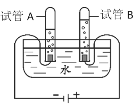
��1���ӿ�����ɽǶȣ�ijͬѧ���ú��ײⶨ�����������ĺ�����ʵ��װ����ͼ1��ʾ��
��ʵ��ԭ����___________���û�ѧ����ʽ��ʾ����
��ʵ�鲽�裺���õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ�
������ʵ����۴�������ֵ�����ܵ�ԭ����_______��
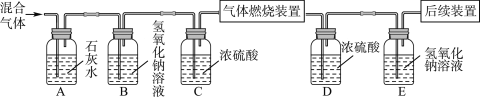
A��װ��©�� B��ʵ��ʱʹ�õĺ�����
C. ʵ��ʱ����ȼ�ճ��ٶ�̫��������ƿ�ڲ��ֿ��������ݳ�
D. ����Ϩ���û�ȼ���ƿ��ȴ�����̴��ɼ�
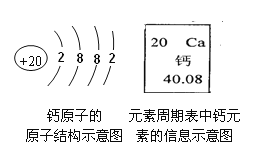
��2�����۽Ƕȣ��á�![]() ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ���![]() ����ʾ��ԭ��
����ʾ��ԭ��

���á�![]() ���ɱ�ʾ������_____�������ƣ���
���ɱ�ʾ������_____�������ƣ���
��ͬ��ͬѹ�£����������ȵ��ڷ�����Ŀ�ȡ��������������ɷֺ��Բ��ƣ�ͼ2�ɱ�ʾ������ģ�͵���_____�����ţ���
��3����Ӧ�ýǶȣ����ÿ�����ȡ���ʵ�����ͼ3��ʾ��
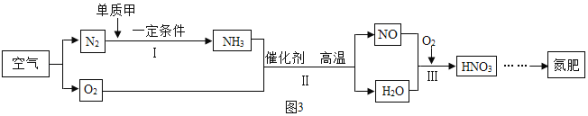
�٢��вμӷ�Ӧ�ĵ��ʼ�Ϊ_____���ѧʽ����
�ڢ� �з�Ӧ�Ļ�ѧ����ʽΪ_____________________��
����������Ԫ�ػ��ϼ۷����仯�ķ�Ӧ��Ϊ������ԭ��Ӧ����Ӧ��_____������ڡ������ڡ���������ԭ��Ӧ��
��4���ӻ����Ƕȣ����б���������ʩ��������_____�����ţ���
A������ͨ���Ӹ��̴�ֱ���ŷŷ���
B���ƹ�ʹ���Ҵ����ʹ�����ͨ����
C���ᳫ���С������г��ȡ���̼�����з�ʽ
���𰸡�4P+5O2![]() 2P2O5 C ������ C H2 4NH3+5O2
2P2O5 C ������ C H2 4NH3+5O2 4NO+6H2O ���� BC
4NO+6H2O ���� BC
��������
��1���ٺ��ײⶨ�����������ĺ���ԭ���Ǻ����ڿ�����ȼ�գ����������ģ����ڼ���ƿ�������ѹǿ��ʽ��뼯��ƿ��ˮ�������Ϊ����������������û�ѧ����ʽ��ʾΪ��4P+5O2![]() 2P2O5����A��װ��©���ᵼ�½��뼯��ƿ��ˮ��������٣��������������ƫС��B���������ᵼ���������ĵĸ���֣�ʹ�����ȷ�����ᵼ������������C��ʵ��ʱ����ȼ�ճ��ٶ�̫��������ƿ�ڲ��ֿ��������ݳ��ᵼ�½��뼯��ƿ��ˮ�������࣬���������������ƫ��D������Ϩ���û�ȼ���ƿ��ȴ�����̴��ɼУ��ᵼ�½��뼯��ƿ��ˮ��������٣��������������ƫС���ʱ���ѡC��
2P2O5����A��װ��©���ᵼ�½��뼯��ƿ��ˮ��������٣��������������ƫС��B���������ᵼ���������ĵĸ���֣�ʹ�����ȷ�����ᵼ������������C��ʵ��ʱ����ȼ�ճ��ٶ�̫��������ƿ�ڲ��ֿ��������ݳ��ᵼ�½��뼯��ƿ��ˮ�������࣬���������������ƫ��D������Ϩ���û�ȼ���ƿ��ȴ�����̴��ɼУ��ᵼ�½��뼯��ƿ��ˮ��������٣��������������ƫС���ʱ���ѡC��
��2������![]() ����ʾ��ԭ�ӣ�����
����ʾ��ԭ�ӣ�����![]() ���ɱ�ʾ�����ǵ����ӣ�����
���ɱ�ʾ�����ǵ����ӣ�����![]() ����ʾ��ԭ�ӣ�����
����ʾ��ԭ�ӣ�����![]() ����ʾ�����ӣ���������ռ������������֮һ�����������뵪���Ӹ���֮��Ϊ1��4���ʱ���ѡC��
����ʾ�����ӣ���������ռ������������֮һ�����������뵪���Ӹ���֮��Ϊ1��4���ʱ���ѡC��
��3�����ɷ�Ӧ����ͼ��֪�������ͼ�Ӧ���ɰ������ʢ��вμӷ�Ӧ�ĵ��ʼ�Ϊ��������ѧʽΪH2�����ɷ�Ӧ����ͼ��֪�����з�ӦΪ������������Ӧ����һ��������ˮ���ʷ�Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2 4NO+6H2O������������Ԫ�ػ��ϼ۷����仯�ķ�Ӧ��Ϊ������ԭ��Ӧ����Ӧ������������Ԫ�ػ��ϼ�Ϊ�㣬��������������Ԫ�ػ��ϼ�Ϊ-2���ʸ÷�Ӧ����������ԭ��Ӧ��
4NO+6H2O������������Ԫ�ػ��ϼ۷����仯�ķ�Ӧ��Ϊ������ԭ��Ӧ����Ӧ������������Ԫ�ػ��ϼ�Ϊ�㣬��������������Ԫ�ػ��ϼ�Ϊ-2���ʸ÷�Ӧ����������ԭ��Ӧ��
��4��A������ͨ���Ӹ��̴�ֱ���ŷŷ��������ܼ����к�������ŷţ�����ɻ�����Ⱦ��ѡ�����B���ƹ�ʹ���Ҵ����ʹ�����ͨ���ͣ��ܼ����к�������ŷţ������ڱ���������ѡ����ȷ��C���ᳫ���С������г�������̼�����з�ʽ���ܼ����к�������ŷţ������ڱ���������ѡ����ȷ���ʱ���ѡBC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ijͬѧΪ�˲ⶨʵ�������������Ʒ������ص�����������ȡ15�˸���Ʒ��5�˶������̻�ϡ����ȸû����T1ʱ��������ʲ��μӷ�Ӧ������ȴ������ʣ�������������ظ����ϲ��������γƵü���T2��T3��T4ʱ���ʣ��������������¼���±���
����ʱ�� | T1 | T2 | T3 | T4 |
ʣ������������ˣ� | 18.2 | 16.6 | 15.2 | 15.2 |
��1����ȫ��Ӧ�����������������____________ ��
��2�������������Ʒ������ص�����������Ҫ����д������̣��������0.1%��___________��