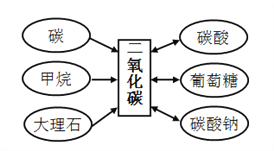
��Ŀ����
����Ŀ����ѧ��Ӧ�����Ǻ�����ѧ��Ӧ���п�������������Ϊ��̽��Ӱ�컯ѧ��Ӧ���ʵ����أ��Թ�������ֽ�Ϊ�о��������ʵ�顣��С����ɲ�����ͼװ�ý���ʵ�飬��¼�ռ�10mL������ʱ�䣬ʵ���ҿɹ�ѡ����Լ��У�2.5%��5%��10%����Ũ�ȵ�H2O2��Һ��MnO2��CuO����ש��ĩ��

A�飺̽�������Ի�ѧ��Ӧ���ʵ�Ӱ�죻
ȡ5mL10%H2O2��Һ������ʵ��ֱ����0.5gMnO2����ש��ĩ��CuO����ʵ�飬��¼�������£�
�Լ� | MnO2 | ��ש��ĩ | CuO |
t/s | 10 | 60 | 20 |
�ɴ˵ó����ۣ�MnO2��CuO��H2O2�ֽ��д����ã���ש��ĩ�����á�
(1)����Ϊ�ý����Ƿ���ȷ��Ϊʲô��_________________________________________��
(2)��������Ա�ʵ�飬֤����ש��ĩ�д����ã������ߵĴ�Ч���ɸߵ��͵�˳��Ϊ___________��
B�飺̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ��
��ͬѧ��ȡ10mL5%H2O2��Һ������0.5gMnO2Ϊ����,������ʵ�飬��¼ʱ��t1��
(3)��ͬѧ��ȡ10mL2.5%H2O2��Һ������__________Ϊ����������ʵ�飬��¼ʱ��t2��
ʵ������_______________________________________________________
���ۣ�___________________________________________________________
����չ̽����
(4)C�飺̽��____________�Ի�ѧ��Ӧ���ʵ�Ӱ�졣
��ȡ10mL5%H2O2��Һ��0.5gCuOΪ��������ʵ�飬����ʵ��ֱ��Թ����ڱ�ˮԡ��50����ˮԡ����¼ʱ��t3��t4��
(5)ʵ������_________________________��
���ۣ�����Ӧ��Ũ�Ⱥʹ�����ͬʱ��__________________________________��
(6)��������ʵ��˵����Ӱ�컯ѧ��Ӧ���ʵ������У�________________________��
���𰸡� ����ȷ�����Ƚϼ����ש��ĩ���������ķֽ������벻�Ӻ�ש��ĩʱ��������ķֽ����ʵĴ�С ![]()
![]()
![]() ��ͬ�����£�H2O2��Ũ��Խ�ֽ�����Խ�� �¶�
��ͬ�����£�H2O2��Ũ��Խ�ֽ�����Խ�� �¶� ![]() �¶�Խ�ߣ�
�¶�Խ�ߣ�![]() �ֽ�����Խ�� ������Ũ�ȡ��¶�
�ֽ�����Խ�� ������Ũ�ȡ��¶�
��������(1)���Ƚϼ����ש��ĩ���������ķֽ������벻�Ӻ�ש��ĩʱ��������ķֽ����ʵĴ�С��(2)�ռ�������������������ʱ��Խ�̣�˵����ѧ��Ӧ����Խ�졣(3)���Ա�ʵ�飬������Ʊ�������ֻ��һ��������ͬʱ���ſ�̽������������ʵ���Ƿ���Ӱ�졣��Ӧ���Ũ��Խ��Ӧ����Խ�졣(4)���������Ϣ��֪����̽���¶ȶԷ�Ӧ���ʵ�Ӱ�졣(5)�¶�Խ�ߣ�H2O2�ֽ�����Խ��(6)Ӱ�컯ѧ��Ӧ���ʵ������д�����Ũ�ȡ��¶ȡ�

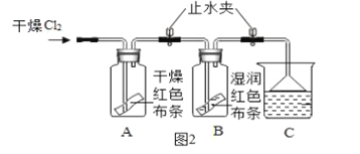
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�����Ŀ��������ͼ�ش�A��B�������е�һ�飬����ȫ�����𣬰�����һ��Ƿ֡�
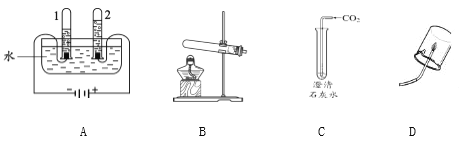
A | B |
��1��Aʵ����Թ�1�в�����������_______�� ��2��B��������������Ӧ�Ļ�ѧ����ʽΪ_____�� | ��1��C�й۲쵽��ʵ��������______�� ��2��Dʵ��ó��Ľ�����_______�� |