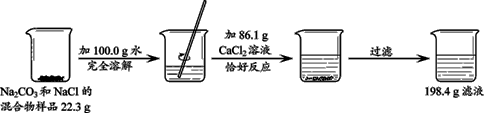��Ŀ����
��2011���Ĵ��ϳ䣬18�⣩ij��ѧ��ѧ����С���ij�����ŷŵĺ���������ķ�ˮ���г�����⡣ȡһ�����ķ�ˮ������������Ϊ1%������������Һ�кͣ��������ʲ�������������Ӧ������ǡ���к�ʱ����������������Һ������Ϊ171g�����˺����Һ������Ϊ268.67g���������ǹ���ʱ��Һ������ʧ����������������λС����
�ٷ�Ӧ�����ɳ���������Ϊ���٣�
�ڸù����ŷŵķ�ˮ�������������������Ϊ���٣�
�ٷ�Ӧ�����ɳ���������Ϊ���٣�
�ڸù����ŷŵķ�ˮ�������������������Ϊ���٣�
�⣺�����ɳ���������Ϊx
Ba��OH��2+ H2SO4 =BaSO4��+2H2O
171 98 233
171��1% y x
171:233=171��1%:x
��ã�x=2.33g
�ڸ��������غ㶨��֪��ȡ��ˮ��Ʒ����Ϊ268.67g+2.33g-171g=100g
���ˮ��Ʒ��������������Ϊy
171:98=171��1%:y
��ã�y=0.98g
����,�ó��ŷŵķ�ˮ���������������Ϊ0.98g��100g��100%=0.98%
����
Ba��OH��2+ H2SO4 =BaSO4��+2H2O
171 98 233
171��1% y x
171:233=171��1%:x
��ã�x=2.33g
�ڸ��������غ㶨��֪��ȡ��ˮ��Ʒ����Ϊ268.67g+2.33g-171g=100g
���ˮ��Ʒ��������������Ϊy
171:98=171��1%:y
��ã�y=0.98g
����,�ó��ŷŵķ�ˮ���������������Ϊ0.98g��100g��100%=0.98%
����
��

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
�����Ŀ

 CaCO3�� + 2NaCl
CaCO3�� + 2NaCl