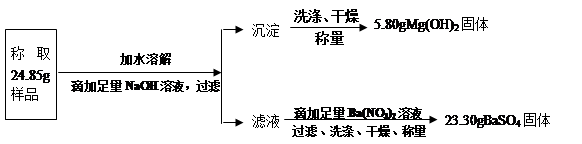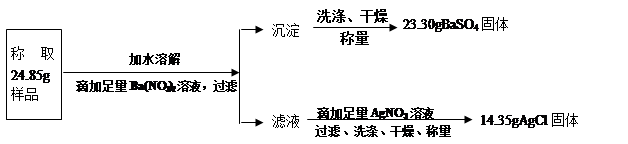��Ŀ����
��10����37��.2009��12��14�գ����о����˳��п�ѧ֪ʶӦ����̽�����ܱ��������е�Ҷ����ǩ������Ŀ����ѡ���ڹ涨ʱ���ڣ������Լ��Ĵ�����ƣ���Ҷ������Ⱦɫ���滭���øƴ�ӵ������ӹ�������ʱij��ѡ��������200��10%������������Һ���������������Ƕ�ʣ����������Ʒ�Һ������������кʹ�����

��1������������Һʱ��Ҫ�������ƹ��� __ __ __ �ˣ�
��2����ȫ�кͷ�Һʱ��ȥ14.6%������100�ˣ���ʣ���Һ�к������������ʶ��ٿˣ�
�������Һ�е��������ʾ��������ᷢ����Ӧ��

��1������������Һʱ��Ҫ�������ƹ��� __ __ __ �ˣ�
��2����ȫ�кͷ�Һʱ��ȥ14.6%������100�ˣ���ʣ���Һ�к������������ʶ��ٿˣ�
�������Һ�е��������ʾ��������ᷢ����Ӧ��
��1��20�����ݴ�����ȷ��λ��Ҳ�ɵ÷֣��������������������� 2��
��2���⣺��ʣ���Һ�к�������������x
NaOH+HCl=NaCl+H2O
40 36.5
x 100�ˡ�14.6%
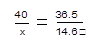 ������������������������1��
������������������������1��
X=16�ˡ���������������������������������1��
�� ��������в�����λ���۷֣�
��ʣ���Һ�к�������������16��
��2���⣺��ʣ���Һ�к�������������x
NaOH+HCl=NaCl+H2O
40 36.5
x 100�ˡ�14.6%
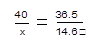 ������������������������1��
������������������������1��X=16�ˡ���������������������������������1��
�� ��������в�����λ���۷֣�
��ʣ���Һ�к�������������16��
��

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ