��Ŀ����
���꣬���ݡ��Ӵ��ס��¼��ٴ����������Ƕ�ʳƷ��ȫ��������Ⱦ�����˼����С��ͬѧ�������ӵ������Ϣ������Ԫ��λ�ڵ������ڢ���塣����������ɫ�й���Ľ������۵�320.9�棬�е�765�棬�ܶ�8.64g/cm3�������Ժ���չ�ԣ����ڳ�ʪ�����л���������ʧȥ���������ӿ������ᣬ�������ڼ���Ӷ�����ʮ���к��������������Ⱦ��ʳƷ��ˮ��������κ����༲�����ش��������⣺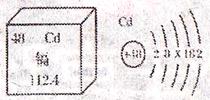
��1������ͼ�е������Ϣ�����ж���Ԫ�ص��жϲ���ȷ����
| A�������ڽ���Ԫ�� | B��һ����ԭ������48������ |
| C����ԭ��ʾ��ͼ��X=18 | D���ӵ����ԭ������Ϊ112.4g |
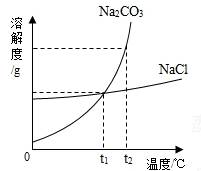
��3�����Ӵ��ס���Ҫ�����ں�ˮ�����������Ⱦ�ĵ������������Ӵ��ס���Ҫ��Դͷ������Ŀǰ��ˮ�����ķ����ǣ�������Na2CO3Ͷ�뱻Cd2+��Ⱦ�ĺ�ˮ�У��γ�CdCO3�������Խ��ͺ�ˮ��Cd2+��Ũ�ȡ�
����ij�Ӷ���Ҫ�����Ȼ��ӣ���̼���Ʒ�Ӧ�Ļ�ѧ����ʽΪ ��
��25��ʱ��CdCO3���ܽ��Ϊ2.8��10-6g����1L��ˮ�к�Cd2+������Ϊ mg����ˮ�ܶ�Ϊ1.0g/cm3����������ȷ��0.001mg��������Ҫ��ˮ��Cd2+�ĺ����ı��Q0.005mg/L����Na2CO3�����ĺ�ˮ ����ǡ�����ꡣ
��1��D��1�֣�
��2�����ڳ�ʪ�����л����������ӿ������ᣬ�����ڼ���ж��ԣ����ٴ�����㣬1�֣�
��3����CdCl2+Na2CO3=CdCO3��+2NaCl����1�֣�����
��0.018��1�֣� ��1�֣�
����

��ϰ��ϵ�д�
�����Ŀ
��H��O��C��Cu����Ԫ����ѡ���ʵ�Ԫ�أ��û�ѧ������գ�ÿ��ֻдһ�֣���
| 2��̼ԭ�� | �������� | ���嵥�� | ������ΪҺ��������� |
| | | | |
 ����ʾһ����ԭ�ӣ���
����ʾһ����ԭ�ӣ��� ����ʾ�������� ����
����ʾ�������� ����
 ���ĵ�����Ϊ�� ����
���ĵ�����Ϊ�� ����