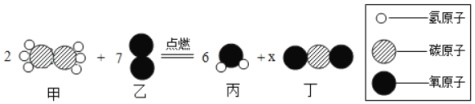
��Ŀ����
����Ŀ��ʵ�������Ʊ��ͳ���ʯ��ˮ����������Ϊ10%������������Һ���������й�ʵ�顣
�±���20��ʱ�������ʵ��ܽ�����ݡ�
���� | Ca(OH)2 | NaOH | CaCO3 | Ca(HCO3)2 | Na2CO3 | NaHCO3 |
�ܽ��/g | 0.16 | 109 | 0.0065 | 16.6 | 21.8 | 9.6 |
(1)������Һ����������100g10%����������Һ�Ļ��������ǣ�
��ȡ�������ƹ��塪��ȡˮ���ܽ⡪װƿ����ǩ��

�ٳ�ȡ�������ƹ��������_____________g��
����֪ˮ���ܶ�Ϊ1g/cm3����100mL��Ͳ��ȡ�����ˮ��_______����ˮ��Һ�档
��װƿ������ǩ���ڱ�ǩ����д��_______________________________��
(2)��������̽��ʵ�飬20��ʱ���������ݻش���������:
���ͳ���ʯ��ˮ��ͨ��CO2ֱ��������������CaCO3����ת��ΪCa(HCO3)2���ɹ۲쵽��������________________________________��
����10%����������Һ��ͨ��CO2ֱ��������������Na2CO3����ת��ΪNaHCO3,�ɹ۲쵽��������_______________________________________________________��
������������4.4gCO2���豥�ͳ���ʯ��ˮ����������Ϊ___________g������10%����������Һ����������Ϊ__________g��(��������ȷ����λ)
���𰸡�10.0  �������� 10% �Ȳ�����ɫ���ǣ�����ͨ������̼����Һ�ֱ���� һ��ʱ�����ְ�ɫ���� 4632 80
�������� 10% �Ȳ�����ɫ���ǣ�����ͨ������̼����Һ�ֱ���� һ��ʱ�����ְ�ɫ���� 4632 80
��������
������̼����������������![]() ������ˮ��̼��ƺͶ�����̼��ˮ��ת��
������ˮ��̼��ƺͶ�����̼��ˮ��ת��![]()
��������̼���������Ʒ�Ӧ������![]() ��ˮ��������̼��̼���ƺ�ˮ��ת��Ϊ
��ˮ��������̼��̼���ƺ�ˮ��ת��Ϊ![]() ��
��
(1) �ٳ�ȡ�������ƹ��������Ϊ![]() ��
��
����֪ˮ���ܶ�Ϊ1g/cm3����100mL��Ͳ��ȡ�����ˮ��ˮ������Ϊ![]() ����ͼΪ
����ͼΪ ��
��
��װƿ������ǩ���ڱ�ǩ����д�������� 10%��
(2)���ͳ���ʯ��ˮ��ͨ��![]() ֱ��������������̼����������������
ֱ��������������̼����������������![]() ������ˮ��̼��ƺͶ�����̼��ˮ��ת��Ϊ
������ˮ��̼��ƺͶ�����̼��ˮ��ת��Ϊ![]() ��̼�����������ˮ���ʿɹ۲쵽���������Ȳ�����ɫ���ǣ�����ͨ������̼����Һ�ֱ���塣
��̼�����������ˮ���ʿɹ۲쵽���������Ȳ�����ɫ���ǣ�����ͨ������̼����Һ�ֱ���塣
����10%����������Һ��ͨ��CO2ֱ��������������̼���������Ʒ�Ӧ������![]() ��ˮ��������̼��̼���ƺ�ˮ��ת��Ϊ
��ˮ��������̼��̼���ƺ�ˮ��ת��Ϊ![]() ��̼�������ܽ�Ƚ�С���ʿɹ۲쵽��������һ��ʱ�����ְ�ɫ������
��̼�������ܽ�Ƚ�С���ʿɹ۲쵽��������һ��ʱ�����ְ�ɫ������
��������4.4gCO2�����������Ƶ���������Ϊx

![]()
![]()
����4.4gCO2���豥�ͳ���ʯ��ˮ����������Ϊ![]()
����10%����������Һ����������Ϊy
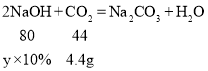
![]()
![]()
����10%����������Һ����������Ϊ80g��