��Ŀ����
����Ŀ����ĸ�춦��1939��3���ں��ϰ���������������������������ĸ�����ƣ�������ʱ����ͭ�Ļ��Ĵ��������ֲ����й����Ҳ���ݡ���ش��������⣺

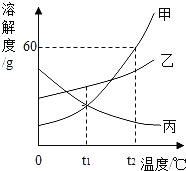
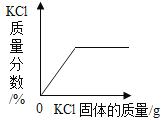
��1����ĸ�춦������ͭ�����Ƴɵģ���ͭ���ϵijɷ���ҪΪͭ���Ͻ�����������������ͭ����______������ĸ�����ŵ㡣
A �����Ժ�
B ���Ժ�
C ��ʴ
��2����ͭ��ͭƬ��Ƚϣ�Ӳ�Ƚϴ����______��
��3����������ұ��ԭ��������ʯ����Ҫ�ɷ�ΪSnO2����ľ̿���ڸ����·�Ӧ���������䷴Ӧ�Ļ�ѧ����ʽ��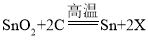 ������X�Ļ�ѧʽΪ______��
������X�Ļ�ѧʽΪ______��
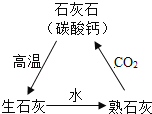
��4�����ٻƽ�����ƭ�˺ܶ��ˣ����ٻƽ�������Ҫ�ɷ���ͭп�Ͻ���ij��ѧ��ȤС���������ٻƽ�������ȡ����ͭ��Cu�������õ�����п��Һ������Ҫ���������ͼ��ʾ����Ӧ��������ȥ��
��֪��![]()
����������������������______��
�����������������������Ӧ�Ļ�ѧ����ʽΪ______��
���𰸡�C ��ͭ CO ���� Zn+H2SO4=ZnSO4+H2��
��������
��1������������������ͭ������ʴ���ŵ㡣���C��
��2����ͭ��ͭƬ��Ƚϣ�Ӳ�Ƚϴ������ͭ�������ͭ��
��3�����ݷ�Ӧǰ��ԭ�ӵ�����䣬ԭ�ӵ���Ŀ���䡣��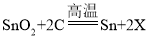 ��֪����Ӧǰ����ԭ�Ӷ���1������Ӧǰ��ԭ�ӡ�̼ԭ�Ӷ���2������Ӧ��Ӧ�ö���2����������2X�У����X�Ļ�ѧʽΪCO�����CO��
��֪����Ӧǰ����ԭ�Ӷ���1������Ӧǰ��ԭ�ӡ�̼ԭ�Ӷ���2������Ӧ��Ӧ�ö���2����������2X�У����X�Ļ�ѧʽΪCO�����CO��
��4�������������ǹ�Һ���룬��˲����������ǹ��ˡ�������ˡ�
�������������������������Ϊ����C�к��й�����п��п��ϡ���ᷴӦ��������п����������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4�TZnSO4+H2�������Zn+H2SO4�TZnSO4+H2����

����Ŀ��ʵ�������Ʊ��ͳ���ʯ��ˮ����������Ϊ10%������������Һ���������й�ʵ�顣
�±���20��ʱ�������ʵ��ܽ�����ݡ�
���� | Ca(OH)2 | NaOH | CaCO3 | Ca(HCO3)2 | Na2CO3 | NaHCO3 |
�ܽ��/g | 0.16 | 109 | 0.0065 | 16.6 | 21.8 | 9.6 |
(1)������Һ����������100g10%����������Һ�Ļ��������ǣ�
��ȡ�������ƹ��塪��ȡˮ���ܽ⡪װƿ����ǩ��

�ٳ�ȡ�������ƹ��������_____________g��
����֪ˮ���ܶ�Ϊ1g/cm3����100mL��Ͳ��ȡ�����ˮ��_______����ˮ��Һ�档
��װƿ������ǩ���ڱ�ǩ����д��_______________________________��
(2)��������̽��ʵ�飬20��ʱ���������ݻش���������:
���ͳ���ʯ��ˮ��ͨ��CO2ֱ��������������CaCO3����ת��ΪCa(HCO3)2���ɹ۲쵽��������________________________________��
����10%����������Һ��ͨ��CO2ֱ��������������Na2CO3����ת��ΪNaHCO3,�ɹ۲쵽��������_______________________________________________________��
������������4.4gCO2���豥�ͳ���ʯ��ˮ����������Ϊ___________g������10%����������Һ����������Ϊ__________g��(��������ȷ����λ)
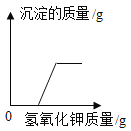
����Ŀ��ij��ѧ��ȤС���ͬѧȡ�������ƺ�̼���ƵĻ����Һ50gװ���ձ��У�ÿ�εμ�50gϡ�����ַ�Ӧ����ò������ݼ�ͼ�����£�
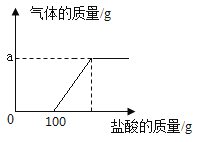
���� | 1 | 2 | 3 | 4 | 5 |
����ϡ���������/g | 50 | 50 | 50 | 50 | 50 |
�ձ������ʵ�����/g | 100 | 150 | 197.8 | 245.6 | 295.6 |
������й���Ϣ���㣺
��1��a����ֵΪ________��
��2��ϡ���������ʵ���������Ϊ_____����д��������̣�
��3��ǡ����ȫ��Ӧ��������Һ�����ʵ�����Ϊ_____����д��������̣�
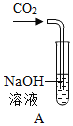
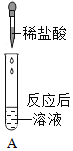
����Ŀ���Ա�ʵ���ǻ�ѧ�о��о������õķ�������ѧ��ȤС���ͬѧΪ�о�CO2ͨ��NaOH��Һ�Ƿ����˷�Ӧ,���������ʵ�顣��ͻ�ѧ��ȤС���ͬѧһ��̽�����ش����⣺
�������ʵ�飩
ʵ�� | ʵ�鲽��һ | ʵ�鲽��� | ʵ�� | ʵ�鲽��һ | ʵ�鲽��� |
ʵ��I |
|
| ʵ��II |
|
|
��̽������ۣ�
(1)ʵ��I�в���һ������Ӧ�Ļ�ѧ����ʽ��____________________________�������������������________________��
(2)ʵ�����в����������Ӧ�Ļ�ѧ����ʽ��____________________________��
CaCl2��ҺҲ������______(�����)����ﵽʵ��Ŀ�ġ�
A���ᱵ��Һ B�Ȼ�����Һ
C̼�����Һ D��������Һ
(3)ʵ�������,����ͬѧ�������ͨ������CO2��NaOH��Һ�еμ���ɫ��̪,�ж������Ƿ�����Ӧ��������С��ͬѧ��,ԭ����____________________________��
���������ϣ�
���� | �ܽ�ȣ�s��/g |
NaOH | 17.3 |
Na2CO3 | ��0.01 |
(4)ͬѧ���ڲ������Ϻ��������ʵ��������CO2ͨ��NaOH�ľƾ���Һ�����۲쵽____________������֤������ȷʵ�����˷�Ӧ��