��Ŀ����
����Ŀ���±���![]() ��
��![]() �ڲ�ͬ�¶�ʱ���ܽ�ȣ��ش����⡣
�ڲ�ͬ�¶�ʱ���ܽ�ȣ��ش����⡣
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g |
| 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
| 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110.0 | |
I.���������У��ܽ�����¶�Ӱ��仯�ϴ����_____��
II.��ȥ![]() ��������
��������![]() �������ǣ���ˮ�ܽ⣬����Ũ����_____�����ˣ�ϴ�ӣ����
�������ǣ���ˮ�ܽ⣬����Ũ����_____�����ˣ�ϴ�ӣ����
III.50��ʱ�����������ʵı�����Һ��100g���ֱ��������10gˮ���ٻָ���50�棬ʣ����Һ��������![]() ��Һ_____������ڡ��������ڡ���С�ڡ���
��Һ_____������ڡ��������ڡ���С�ڡ���![]() ��Һ��
��Һ��
IV.![]()

A����Һ_____������͡������͡�����Һ��C����Һ����������_____g��
���𰸡�![]() �����ᾧ ���� ������ 167.6
�����ᾧ ���� ������ 167.6
��������
I���ɱ��е����ݿ�֪�����������У��ܽ�����¶�Ӱ��仯�ϴ����KNO3�����KNO3��
II������ص��ܽ�����¶ȱ仯Ӱ��ϴ��Ȼ��Ƶ��ܽ�����¶�Ӱ���С�����Գ�ȥ�Ȼ���������������أ������ǣ���ˮ�ܽ⣬����Ũ���������ᾧ�����ˣ�ϴ�ӣ������������ᾧ��
III���ɱ��е����߿�֪����50��ʱ��KNO3�ܽ�ȴ���NaCl���ܽ�ȣ�����50��ʱ�����������ʵı�����Һ��100g���ֱ��������10gˮ���ٻָ���50�棬KNO3�����ľ���࣬ʣ����Һ��������NaCl��Һ����KNO3��Һ��������ڡ�
IV���ɱ��е����ݿ�֪����60��ʱ��KNO3���ܽ����110g����A��Һ��100g��ˮ���ܽ���40g��KNO3���γɵ���ҺΪ��������Һ���ڽ��µ�20��ʱ��100g��ˮ�����ܽ�31.6g��KNO3��36g��NaCl�����γɵ���Һ������Ϊ167.6g����������ͣ�167.6��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
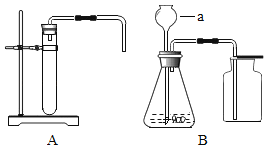
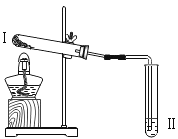
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ʵ����ѡ����ͼ��ʾװ����ȡ���壬����Ҫ��ش��������⡣
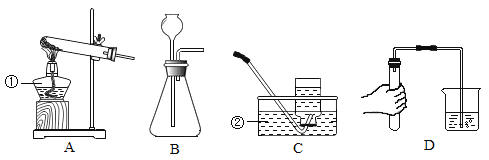
��1��ָ����ŵ��������Ƣ�_______����_______��
��2��ʵ������Bװ����ȡ������̼�Ļ�ѧ����ʽ��_______________________��
װ��B����������ȡ��������___________________________��
��3��ͼD��ʵ�����Ŀ����________________________________________��
��4��ʵ���ҳ��ü����Ȼ�狀���ʯ�ҵĹ���������ȡ������
�� Ŀ | ���� | ���� | ������̼ | ���� |
0����101kpaʱ���ܶȣ�g/L�� | 1.293 | 1.429 | 1.977 | 0.771 |
20����101kpaʱ1���ˮ���ܽ��������� | �M | 0.031 | 1 | 700 |
����װ�ÿ�ѡ��_______������ţ��������ϱ����ݿ�֪ʵ�����ռ�����_______��������������������ѡ��Cװ�á�
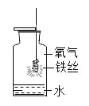
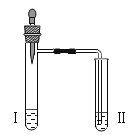
����Ŀ������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ��ǣ� ��
ʵ�� | A | B | C | D |
װ�� |
|
|
|
|
���� | ����ƿ�е�ˮ���� �շų������� | ��Ͳ�е�ˮ��ͨ��ˮ ������仯�ó� O2 ����� | ����ƿ�е�ˮ���� ȴ����������� ֹ����ƿը�� | ����ƿ�е�ˮ��ˮ �Ƚ�����ƿ�ڵĿ� ���ž� |
A. A B. B C. C D. D
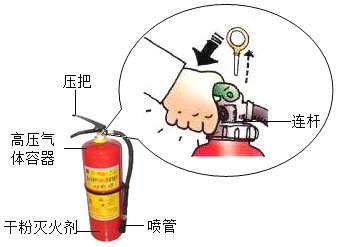
����Ŀ��С����СӢͬѧ����:�����ǵ���ǩ�ڿ����в��Ḵȼ������װ��60%ˮ�ļ���ƿ������ˮ�����ռ��������õ���������ʹ�����ǵ���ǩ��ȼ��Ϊ���ҵ���ʹ��������ǩ��ȼ������������Сֵ�����ǽ���������̽��:
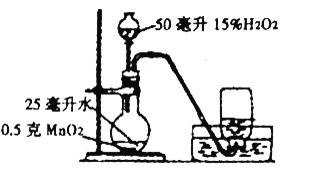
�����ʵ�飩С���������ͼ��װ�ã���װ�õ�Բ����ƿ�з���25����ˮ��Ŀ����__________��
��ʵ���о�����һ��ʵ��:ȡ3ֻ����ƿ�����Ϊ�٢ڢ۷ֱ�װ��![]() ��
��![]() ��
��![]() ��ˮ�����ϸDz�Ƭ������ˮ���С�������__________�ų����ܿ����뼯��ƿ�����е�ˮȫ���ų�������ͬ��3֧��������ǩ�ֱ�����-�ۺ�ƿ�У���¼ʵ������
��ˮ�����ϸDz�Ƭ������ˮ���С�������__________�ų����ܿ����뼯��ƿ�����е�ˮȫ���ų�������ͬ��3֧��������ǩ�ֱ�����-�ۺ�ƿ�У���¼ʵ������
��õ�һ��ʵ�������С�������˵ڶ���ʵ�飬����¼ʵ����������ʵ�����ݺ�������±�:
ʵ����� | ��һ�� | �ڶ��� | ||||
ʵ����� | �� | �� | �� | �� | �� | �� |
����ƿװˮ���������/% | 15 | 30 | 45 | 34 | 38 | 42 |
��������ǩ״�� | �� | ���� | ��ȼ | ���� | ��ȼ | ��ȼ |
�ó���������ʵ�������СӢ��Ϊ������ȷ����ʹ��������ǩ��ȼ������������Сֵ�����������ʵ�飬��ʵ����Ţߵļ���ƿװˮ���������Ӧ����__________%����ʱ��ļ���ƿ���������������Ϊ__________%��
��ʵ����ۣ������ʵ��ó�:��ʹ��������ǩ��ȼ������������СֵΪ![]() ��
��
������̽����С����Ϊ���ø÷����ռ��������к���һ������__________���������ʵ��Ľ��Ĵ�ʩ__________��
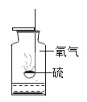
����Ŀ��ʵ�����ÿ�״����ʯ��5%��ϡ���ᷴӦ��ȡ![]() ���۲첻�����ݲ���ʱ�����ֹ��廹��ʣ�࣬��ѧ��ȤС���ͬѧ�Դ˽���̽�������ʲ�����ˮ�Ҳ����ᷴӦ����
���۲첻�����ݲ���ʱ�����ֹ��廹��ʣ�࣬��ѧ��ȤС���ͬѧ�Դ˽���̽�������ʲ�����ˮ�Ҳ����ᷴӦ����
��������⣩���ٲ������ݵ�ԭ����ʲô��
��������裩����һ�������Ѿ���ȫ��Ӧ
�������ʣ���������̼���
������������������ʸ����ڴ���ʯ���棬�谭��Ӧ��������
��ʵ��̽��һ����Ӧ����Һ���Ƿ������ᡣ
ʵ�鲽�� | ���� | ���� |
ȡ������Ӧ�����Һ������ҺpH | �����Һ | ˵����Һ��_____�ԣ��ɴ˵ó�����һ�������Ľ��ۡ� |
�����ɣ���ͬѧ��Ϊ�˽��۲����Ͻ�����Ӧ�������������Һ�����ǿ����Ӱ�졣
������ʵ�飩
��ȡ�ɾ����ձ���������_____��Һ�������Һ![]() ��
��
��ȡ�ྻ���Թܣ���������ˮ����ɫʯ����Һ������ͨ��![]() ��������Һ��Ϊ_____ɫ��
��������Һ��Ϊ_____ɫ��
����ȡ�ྻ���Թܣ���������ˮ������ͨ��![]() �����ͣ������Һ
�����ͣ������Һ![]() ��
��
������������ʵ���У��ܶ����������������͵���_____����ʵ���ţ����ɴ˿�֪��̽��һ�������ﲻ��ʹ��Һ��_____������һ����������Ӧ�����Һ�������ᡣ
��ʵ��̽������ʣ��������Ƿ���̼��ơ�
ʵ�鲽�� | ���� | ���� |
����Ӧ������ʹʣ������Һ���ֽӴ� | �������ݲ��� | ���ۣ�����_____�������� |
���������еμ�����5%��ϡ���� | �������ݲ����������ٲ�������ʱ�����й���ʣ�� |
�ۺ�̽��ʵ��һ��̽��ʵ������ɵó�Ӱ�췴Ӧ�ܷ���е�����֮һ��_____��
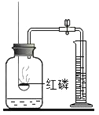
����Ŀ��̼���ƺ�̼�������������г������Σ�ͨ��ʵ����֤��̽�����ǵĻ�ѧ���ʣ�
���������ϣ�
��Na2CO3+CaCl2�TCaCO3��+2NaCl
��2NaHCO3![]() Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
��Ca��HCO3��2 ������ˮ��
��CaCl2 ��Һ�ֱ��� NaHCO3��Na2CO3 ��Һ�������������еİٷ���Ϊ��Һ�����ʵ�������������
NaHCO3 | Na2CO3 | ||||
0.1% | 1% | 5% | 0.1% | ||
CaCl2 | 0.1% | ���������� | ��� | ��� | ��� |
1% | ���������� | �л��� | �л��ǣ���С���� | �г��� | |
5% | ���������� | ��� | ������������ | ��� | |
������ʵ�飩
��� | ʵ��װ�� | ��Ҫʵ�鲽�� | ʵ������ |
ʵ�� 1 |
| ��2֧�Թ��зֱ������ ��Na2CO3 �� NaHCO3 ��Һ���ٷֱ�μ����� | 2 |
ʵ�� 2 |
| �����м����Լ�a�������� ��������Na2CO3�� NaHCO3 ���壬�ֱ����һ��ʱ�� | Na2CO3 ����ʱ�������� ������ NaHCO3 ����ʱ���г��ֻ��� |
ʵ�� 3 |
| �����м����Լ� a�������� �������� 5%�� NaHCO3 �� Һ���ٵμ� 5%�� CaCl2 �� Һ | ���г��ֻ��ǣ������ݲ� �����г��ֻ��� |
����������ۣ�
��1��ʵ��1�У�NaHCO3�����ᷴӦ�Ļ�ѧ����ʽΪ_____��
��2��ʵ��2�У��Լ�aΪ_____��
��3��ʵ��3�У�NaHCO3 �� CaCl2 ��Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3+CaCl2�T_____+_____+_____+H2O
����˼�����ۣ�
��1��ʵ�� 2 �У����� NaHCO3���Թ����в�������ɷֿ���Ϊ_____��д�����п��ܣ���
��2�����Ϣ��У�NaHCO3��Һ�� CaCl2 ��Һ��ϵ������У���Щֻ�۲쵽���ǡ�δ�۲쵽���ݣ�ԭ�������_____��
��3����2�ֲ�ͬ�ķ�������Na2CO3��NaHCO3 ���壬ʵ�鷽���ֱ�Ϊ��
��_____��
��_____��