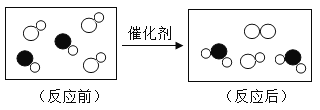
��Ŀ����
����Ŀ���������ƾ��壨CaO28H2O�����ȶ����ʰ�ɫ������ˮ���㷺Ӧ���ڻ���ɱ�����������Ա���Ϊԭ���Ʊ�CaO2�������£�
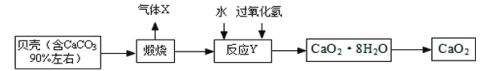
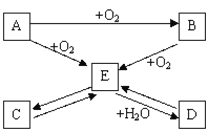
��1������X��______�����������ƾ�������Һ����IJ���������______��
��2����ӦY�ǻ��Ϸ�Ӧ����Ӧ������CaO28H2O����д����ѧ����ʽ______�������ѣ�CaO28H2O�ɿ�����1��CaO2���Ӻ�8��ˮ���ӹ��ɵ�ˮ���
��3���������ƾ��壨CaO28H2O����H��OԪ�ص�������Ϊ_____��
���𰸡�������̼ ���� CaO+H2O2+7H2O�TCaO28H2O 1��10
��������
��1��̼��Ʒֽ����������ƺͶ�����̼������X�������Ƕ�����̼�����������ƾ�������Һ����ķ����ǹ��ˣ�
�ʴ�Ϊ������̼�����ˣ�
��2����ӦY�ǵĻ�ѧ����ʽ�ǣ�CaO+H2O2+7H2O�TCaO28H2O��
�ʴ�Ϊ CaO+H2O2+7H2O�TCaO28H2O��
��3���������ƾ��壨CaO28H2O����H��OԪ�ص�������Ϊ1��16��16��10=1��10��
�ʴ�Ϊ1��10��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����У��ѧ��ȤС��ͬѧΪ�˲ⶨ����ʯ��ʯ��̼��Ƶ��������������Dz��õķ������£�ȡ��ʯ��ʯ��Ʒ16g����80gϡ������Ĵμ��룬���������������ݼ��±�(���ʲ������ᷴӦ��Ҳ������ˮ)��������Ǽ��㣺
��� | ����ϡ���������/g | ʣ����������/g |
��1�� | 20 | 11 |
��2�� | 20 | 6 |
��3�� | 20 | 2.8 |
��4�� | 20 | n |
(1)̼��Ƶ���Է������� _______��
(2)�ϱ���n����ֵ�� _________ ��
(3)��Ʒ��̼��Ƶ��������� _________ ��
(4)���ɶ�����̼������Ϊ__________(д���������)��