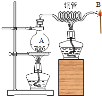
��Ŀ����
����Ŀ��������þ������һ������ϡHCl�У������������ݺ���ֻҰ�ɫ���塣ijͬѧ�Ը�����������̽����(��֪��MgCl2����������ˮ)
(һ)�������ݷ�Ӧ�Ļ�ѧ����ʽ____��
(��)���ˡ�ϴ�ӡ��������ɵûҰ�ɫ���塣�ԻҰ�ɫ���������²²Ⲣ����ʵ�飺
���²⣩�Ұ�ɫ��������ǣ���ʣ���Mg�� ��____�� ��Mg��MgCl2���塣
��ʵ��1��(1)ȡ�Ұ�ɫ������Ʒ������ϡHCl�������ݲ�������²� __(ѡ�����)��������(2)��ȡ�������ĻҰ�ɫ�����MgCl2����ֱ����ͬ�¡�����ˮ�У��Ұ�ɫ���岻�ܽ⣬��MgCl2����ȫ��____����²�ڲ�������
���������ϣ����Ұ�ɫ����ijɷ�Ϊ��ʽ�Ȼ�þ����ѧʽ�ɱ�ʾΪMgx(OH)yClznH2O[��ÿ��Mgx(OH)yClz�����n��H2O]�������ȷֽ�ɲ�������þ���Ȼ����ˮ��
��ʵ��2����ȡ�ûҰ�ɫ����47.5g��������ȷ�IJ�������������ȷֽ�ʵ�飬����Ӧֹͣ�������þ��������Ϊ24.0g��ˮ������16.2g��
���������ۣ�
(1)X��Z=____��
(2)Mgx(OH)yCl�Ļ�ѧʽ������ ______��
(3)������Mgx(OH)yClznH2O�У�n��5����Y����ֵΪ____
���𰸡�Mg+2HCl=MgCl2+H2�� MgCl2���� �١��� �ܽ� X��Z=3:1 Mg3(OH)5Cl 10
��������
(һ)þ�����������Ȼ�þ����������Ӧ�Ļ�ѧ����ʽMg+2HCl=MgCl2+H2����
(��) [�²�]��Ӧ���������һ�����������������ʣ��ķ�Ӧ��Ұ�ɫ��������ǣ���ʣ���Mg�� ��MgCl2���壻 ��Mg��MgCl2���壻
[ʵ��1](1)þ�����ᷴӦ�����������������ݣ��Ȼ�þ�������Ӧ��ȡ�Ұ�ɫ������Ʒ������ϡHCl�������ݲ�������û��þ���²�٢۲�������
(2)þ��������ˮ����ȡ�������ĻҰ�ɫ�����MgCl2����ֱ����ͬ�¡�����ˮ�У��Ұ�ɫ���岻�ܽ⣬��MgCl2����ȫ���ܽ⣬��²�ڲ�������
[ʵ��2] ��ʽ�Ȼ�þ����ѧʽ�ɱ�ʾΪMgx(OH)yClznH2O[��ÿ��Mgx(OH)yClz�����n��H2O]�������ȷֽ�ɲ�������þ���Ȼ���͡���ȡ�ûҰ�ɫ����47.5g��������ȷ�IJ�������������ȷֽ�ʵ�飬����Ӧֹͣ�������þ��������Ϊ24.0g��þԪ�ص�����Ϊ��24.0g��![]() =14.4g��ˮ������16.2g������Ԫ�ص�����Ϊ��16.2g��
=14.4g��ˮ������16.2g������Ԫ�ص�����Ϊ��16.2g��![]() =1.8g �������Ȼ������������Ϊ��47.5g-24.0g-16.2g=7.3g����Ԫ�ص�����Ϊ7.3g��
=1.8g �������Ȼ������������Ϊ��47.5g-24.0g-16.2g=7.3g����Ԫ�ص�����Ϊ7.3g��![]() =7.1g����Ԫ�ص�����=7.3g-7.1g=0.2g��
=7.1g����Ԫ�ص�����=7.3g-7.1g=0.2g��
[��������]
(1)X��Z=![]() ��
��![]() =3:1��
=3:1��
(2) X��Z =3:1����X=3��Z=1ʱ���ɵ�Mg3(OH)yCl����������Ԫ�صĻ��ϼ۴�����Ϊ�㣬��������-1�ۣ�þ��+2�ۣ�����-1�ۣ�����y=5����ѧʽ��Mg3(OH)5Cl��
(3) X��Z =3:1����Z=m����x=3m��������Mgx(OH)yClznH2O�У�n��5��Mgx(OH)yClznH2O�ɱ�ʾΪMg3m(OH)yClm5H2O�������������֪����������Ԫ�ع�2g����Ԫ�ع�7.1g
�ɵã�![]() ��
��
![]()
�ⷽ�����Y����ֵΪ10��

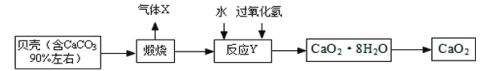
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ȡ10gʯ��ʯ��Ʒ���ձ��У��������м�������ϡ���ᣨ���ʲ���Ӧ�����й�ʵ�����ݼ��±���
��Ӧǰ | ��Ӧ�� | |
�ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ������л��������� |
160g | 10g | 166.7g |
��1��CaCO3��CaԪ�ص���������Ϊ____________��
��2������CO2������____________��
��3����ʯ��ʯ��Ʒ��̼��Ƶ���������____________��д��������̣���