��Ŀ����
����Ŀ�����������������������й㷺Ӧ�á�ʵ��С��Թ��������ijЩ���ʽ����о���
���ȶ���
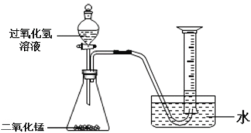
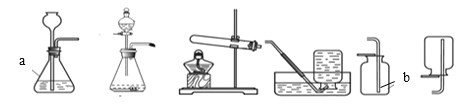
��1������ͼ��ʾ����ʵ�飬��������ֽ�Ļ�ѧ����ʽΪ______��
��2��������ˮ���ռ�O2��ԭ����______��
��3��̽���¶ȶԹ�������ֽ����ʵ�Ӱ�졣
ͬѧ�ǽ��������µ�ʵ�飬ʵ���������±���
ʵ����� | �� | �� | �� |
H2O2��Һ��Ũ�� % | 30 | 30 | 30 |
H2O2��Һ�����/mL | 6 | 6 | 6 |
�¶�/�� | 20 | 35 | 55 |
MnO2������/g | 0 | 0 | 0 |
�ռ�O2�����/mL | 0 | 1.9 | 7.8 |
��Ӧʱ�� | 40 min | 40 min | 40 min |
�ɴ˵ó��Ľ�����______��
��ʴ��
���������ϣ�H2O2��Һ�и�ʴ�ԡ�
������ʵ�飩
ͬѧ����ͭƬ���ʵ����֤H2O2��Һ�ĸ�ʴ�ԡ�������ʵ����H2O2��Һ��ϡ��������Ũ�Ⱦ���ͬ��
��ͭƬ�ֱ������3����Һ�н���ʵ�飬���±���
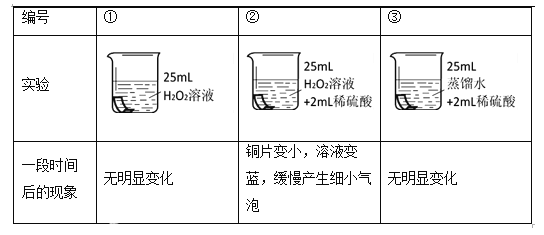
����������ۣ�
��4��ʵ��ٵ�������______��
��5����˵��ͭƬ����ʴ��H2O2��Һ��ϡ������йص�ʵ������______��
��6��ͭƬ����ʴ�ķ�Ӧ���£���ȫ�÷�Ӧ�Ļ�ѧ����ʽ��Cu + H2O2+ H2SO4=== CuSO4 +��_______/p>
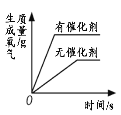
���𰸡�2H2O2![]() 2H2O+O2�� ������������ˮ������������ˮ��Ӧ ������������ͬ������£��¶�Խ�ߣ�H2O2�ֽ�����Խ�� ����ʵ�� �٢ڢۣ���٢ں͢ڢ� �� 2H2O
2H2O+O2�� ������������ˮ������������ˮ��Ӧ ������������ͬ������£��¶�Խ�ߣ�H2O2�ֽ�����Խ�� ����ʵ�� �٢ڢۣ���٢ں͢ڢ� �� 2H2O
��������
��1�����������ڶ�������������������������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����
��2��������ˮ���ռ�O2��ԭ���ǣ�������������ˮ����������ˮ��Ӧ��
��3��̽���¶ȶԹ�������ֽ����ʵ�Ӱ�죬�ɱ���ó��Ľ����ǣ�������������ͬ������£��¶�Խ�ߣ���������ֽ��Խ�죻
��4����ֻ��H2O2��Һû��ϡ���������£��о����������ͭƬ�ĸ�ʴ�ԣ���ʵ������Աȣ�֤��ͭƬ����ʴ��ϡ�����йأ�
��5���Ա�����ʵ��ɵã�ʵ��٢���̽��ͭƬ����ʴ�Ƿ���ϡ�����йأ���ʵ��ڢ�̽��ͭƬ����ʴ�Ƿ���H2O2�йأ�
��6��ͭƬ����ʴ�ķ�Ӧ�ǣ�ͭ���������ϡ���ᷴӦ��������ͭ��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��Cu+H2O2+H2SO4=CuSO4+2H2O��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
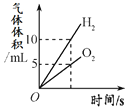
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ������ͼ������ȷ��ӳ��Ӧ�仯��ϵ����
|
|
|
|
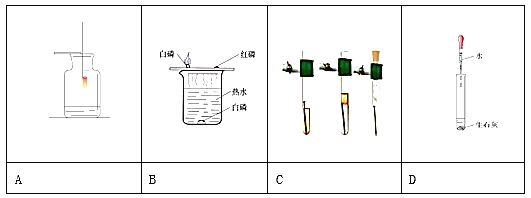
A���õ���������Ũ�ȵ�˫��ˮ��ȡ���� | B�����ú���ȼ�ղⶨ�����������ĺ�������ֹˮ��ǰ�� | C����ˮͨ����һ��ʱ�� | D����һ�����Ķ��������м������������Һ |
A. A B. B C. C D. D