ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΑ±ΦνΖ®…ζ≤ζ¥ΩΦνΒΡ÷ς“ΣΖ¥”Π‘≠άμ»γœ¬:
Θ®1Θ© NaCl + NH3 + CO2 + H2O =NaHCO3 + NH4C1
Θ®2Θ©2NaHCO3![]() Na2CO3 +CO2Γϋ +H2OΓΘΕ‘…œ ω–≈œΔΒΡ”–ΙΊάμΫβ≤Μ’ΐ»ΖΒΡ «
Na2CO3 +CO2Γϋ +H2OΓΘΕ‘…œ ω–≈œΔΒΡ”–ΙΊάμΫβ≤Μ’ΐ»ΖΒΡ «
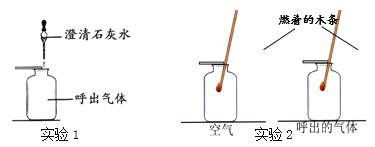
A. Θ®1Θ©÷–Έω≥ωΨßΧεΚσ Θ”ύ»ή“Κ÷–÷Μ”–“Μ÷÷»ή÷
B. Α±―ΈΥ°±» ≥―ΈΥ°Ηϋ“ΉΈϋ ’Εΰ―θΜ·ΧΦ
C. ΗΟΧθΦΰœ¬NaHCO3ΒΡ»ήΫβΕ»±»NH4C1ΒΡ–Γ
D. Έω≥ωΨßΧεΚσΒΡ»ή“ΚΈΣNaHCO3ΒΡ±ΞΚΆ»ή“Κ
ΓΨ¥πΑΗΓΩA
ΓΨΫβΈωΓΩ
AΓΔΘ®1Θ©÷–Έω≥ωΨßΧεΚσ Θ”ύ»ή“Κ÷–”–NaHCO3 ”κNH4ClΝΫ÷÷»ή÷ Θ§―Γœν¥μΈσΘΜBΓΔΑ±―ΈΥ°œ‘Φν–‘Θ§”κΕΰ―θΜ·ΧΦΡήΖ¥”ΠΘ§Υυ“‘±» ≥―ΈΥ°Ηϋ“ΉΈϋ ’Εΰ―θΜ·ΧΦΘ§―Γœν’ΐ»ΖΘΜCΓΔΗΟΧθΦΰœ¬NaHCO3ΒΡ»ήΫβΕ»±»NH4C1ΒΡ–Γ≤≈”–άϊ”ΎΧΦΥα«βΡΤΒΡΫαΨßΘ§―Γœν’ΐ»ΖΘΜDΓΔ±ΞΚΆ»ή“Κ≤≈ΡήΖΔ…ζΫαΨßΘ§Έω≥ωΨßΧεΚσΒΡ»ή“ΚΈΣNaHCO3ΒΡ±ΞΚΆ»ή“ΚΘ§―Γœν’ΐ»ΖΘ§Ι ―ΓAΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ Β―ι–ΓΉιΕ‘Ιΐ―θΜ·«β÷Τ»Γ―θΤχ÷–”ΑœλΖ¥”ΠΥΌ¬ ΒΡ“ρΥΊΫχ––ΧΫΨΩΓΘ
[Χα≥ωΈ Χβ] ΡΡ–©“ρΥΊΩ…Ρή”ΑœλΗΟΖ¥”ΠΒΡΖ¥”ΠΥΌ¬ ΘΩ
[Ής≥ω≤¬œκ] –ΓΖΦΆ§―ßΘΚH2O2»ή“ΚΒΡ≈®Ε»Ω…Ρή”ΑœλΗΟΖ¥”ΠΒΡΖ¥”ΠΥΌ¬ ΘΜ
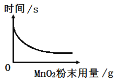
–ΓΜΣΆ§―ßΘΚ¥ΏΜ·ΦΝΘ®MnO2Θ©ΒΡ”ΟΝΩΩ…Ρή”ΑœλΗΟΖ¥”ΠΒΡΖ¥”ΠΥΌ¬ Γ≠Γ≠
[ Β―ι―ι÷Λ]
Θ®1Θ©ΈΣΝΥΧΫΨΩ‘ΎΤδΥϊΧθΦΰœύΆ§ ±H2O2»ή“Κ≈®Ε»Ε‘Ζ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§–ΓΖΦΆ§―ßΒΡ Β―ι «ΘΚΟΩ¥ΈΨυ»Γ10mL30%ΒΡH2O2»ή“ΚΘ§»ΜΚσ≈δ≥…≤ΜΆ§≈®Ε»ΒΡ»ή“ΚΘ§‘ΎœύΆ§Έ¬Ε»œ¬Φ”»κΒ»÷ ΝΩMnO2Ϋχ–– Β―ιΘ§≤βΕ®Ης¥Έ ’Φ·ΒΫ100mL―θΤχ ±Υυ”ΟΒΡ ±ΦδΘ§Φ«¬Φ ΐΨί»γœ¬ΘΚ
Β―ι¥Έ–ρ | 1 | 2 | 3 | 4 | 5 |
H2O2»ή“Κ≈®Ε» | 1% | 5% | 15% | 25% | 30% |
Υυ”Ο ±ΦδΘ®ΟκΘ© | 660 | 205 | 25 | 4 | 3 |
Ά®Ιΐ…œ±μ Β―ι ΐΨίΖ÷ΈωΩ…ΒΟΒΫΒΡΫα¬έ «ΘΚ‘ΎΤδΥϊΧθΦΰœύΆ§ΒΡ«ιΩωœ¬Θ§_____‘Ϋ¥σΘ§Ζ¥”ΠΥΌ¬ ‘Ϋ_____ΓΘ
Θ®2Θ©ΈΣΝΥΧΫΨΩ‘ΎΤδΥϊΧθΦΰœύΆ§ ±¥ΏΜ·ΦΝΘ®MnO2Θ©ΒΡ”ΟΝΩΕ‘Ζ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§–ΓΜΣΆ§―ßΒΡ Β―ι «ΘΚΟΩ¥ΈΨυ”Ο30mL10%ΒΡH202»ή“Κ‘ΎœύΆ§Έ¬Ε»œ¬Ϋχ–– Β―ιΘ§≤…”Ο≤ΜΆ§ΝΩMnO2ΖέΡ©Ήω¥ΏΜ·ΦΝΘ§≤βΕ®Ης¥Έ ’Φ·ΒΫ100mL―θΤχ ±Υυ”ΟΒΡ ±ΦδΘ§ΜφΆΦ»γœ¬ΘΚ
ΔΌΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ__________
ΔΎΆ®Ιΐ»γΆΦ Β―ι ΐΨίΖ÷ΈωΩ…ΒΟΒΫΒΡΫα¬έ «ΘΚ ________
Θ®3Θ©–ΓΟςΆ§―ß»œΈΣΤδΥϊΧθΦΰ“≤Ω…ΡήΜα”ΑœλΗΟΖ¥”ΠΒΡΥΌ¬ ΓΘ
–ΓΟςΆ§―ßΒΡ Β―ι «ΘΚΟΩ¥ΈΨυ»Γ10mL30%ΒΡH2O2»ή“ΚΘ§»ΜΚσ≈δ≥…“ΜΕ®≈®Ε»ΒΡ»ή“ΚΫχ–– Β―ιΓΘ
Β―ι¥Έ–ρ | 1 | 2 | 3 | 4 | 5 |
H2O2»ή“Κ≈®Ε» | ΔΌ | 10% | 10% | 10% | 10% |
Εΰ―θΜ·ΟΧΖέΡ©”ΟΝΩ/g | 0Θ°2 | 0Θ°2 | ΔΎ | 0Θ°2 | 0Θ°2 |
Έ¬Ε»/Γφ | 20 | 30 | 40 | 50 | 60 |
¥ΐ≤β ΐΨί |
Χν–¥±μ÷– ΐΨίΘΚΔΌ ___________ ΔΎ ___________ΓΘ
Δέ–ΓΟςΆ§―ß Β―ιΧΫΨΩΒΡΡΩΒΡ « _________ΓΘ
Δή“‘œ¬¥ΐ≤β ΐΨίΚœάμΒΡ «_____________
AΘ°≤βΕ®Ης¥Έ Β―ι ’Φ·ΒΫ100mL―θΤχ ±Υυ”ΟΒΡ ±Φδ
BΘ°≤βΕ®Ης¥Έ Β―ι‘Ύ30sάο ’Φ·ΒΫΒΡ―θΤχΧεΜΐ
CΘ°≤βΕ®Ης¥Έ Β―ιΆξ»ΪΖ¥”Π ± ’Φ·ΒΫΒΡ―θΤχΧεΜΐ
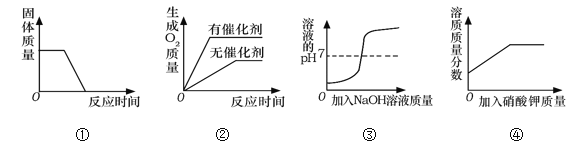
ΓΨΧβΡΩΓΩΙΐ―θΜ·«β‘Ύ…ζ≤ζ…ζΜν÷–”–ΙψΖΚ”Π”ΟΓΘ Β―ι–ΓΉιΕ‘Ιΐ―θΜ·«βΒΡΡ≥–©–‘÷ Ϋχ––―–ΨΩΓΘ
ΔώΘ°≤ΜΈ»Ε®–‘
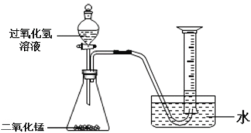
Θ®1Θ©»γ…œΆΦΥυ ΨΫχ–– Β―ιΘ§Ιΐ―θΜ·«βΖ÷ΫβΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
Θ®2Θ©Ρή”Ο≈≈Υ°Ζ® ’Φ·O2ΒΡ‘≠“ρ «______ΓΘ
Θ®3Θ©ΧΫΨΩΈ¬Ε»Ε‘Ιΐ―θΜ·«βΖ÷ΫβΥΌ¬ ΒΡ”ΑœλΓΘ
Ά§―ßΟ«Ϋχ––ΝΥ»γœ¬ΒΡ Β―ιΘ§ Β―ι ΐΨί»γœ¬±μΘΚ
Β―ι–ρΚ≈ | ΔΌ | ΔΎ | Δέ |
H2O2»ή“ΚΒΡ≈®Ε» % | 30 | 30 | 30 |
H2O2»ή“ΚΒΡΧεΜΐ/mL | 6 | 6 | 6 |
Έ¬Ε»/Γφ | 20 | 35 | 55 |
MnO2ΒΡ”ΟΝΩ/g | 0 | 0 | 0 |
’Φ·O2ΒΡΧεΜΐ/mL | 0 | 1.9 | 7.8 |
Ζ¥”Π ±Φδ | 40 min | 40 min | 40 min |
”…¥ΥΒΟ≥ωΒΡΫα¬έ «______ΓΘ
ΔρΘ°Η· ¥–‘
Θ®≤ι‘ΡΉ ΝœΘ©H2O2»ή“Κ”–Η· ¥–‘ΓΘ
Θ®Ϋχ–– Β―ιΘ©
Ά§―ßΟ«”ΟΆ≠Τ§…ηΦΤ Β―ι―ι÷ΛH2O2»ή“ΚΒΡΗ· ¥–‘ΓΘΘ®ΗςΉι Β―ι÷–H2O2»ή“ΚΚΆœΓΝρΥαΥυ”Ο≈®Ε»ΨυœύΆ§Θ©
ΫΪΆ≠Τ§Ζ÷±πΫΰ≈ί‘Ύ3÷÷»ή“Κ÷–Ϋχ–– Β―ιΘ§»γœ¬±μΓΘ
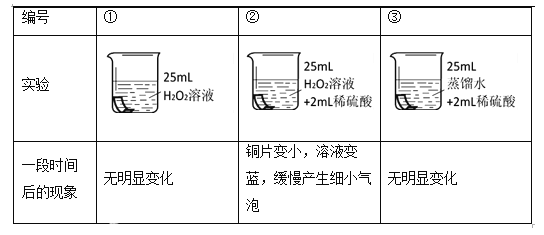
Θ®Ϋβ Ά”κΫα¬έΘ©
Θ®4Θ© Β―ιΔΌΒΡΉς”Ο «______ΓΘ
Θ®5Θ©ΡήΥΒΟςΆ≠Τ§±ΜΗ· ¥”κH2O2»ή“ΚΚΆœΓΝρΥαΨυ”–ΙΊΒΡ Β―ιΉι «______ΓΘ
Θ®6Θ©Ά≠Τ§±ΜΗ· ¥ΒΡΖ¥”Π»γœ¬Θ§≤Ι»ΪΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΓΘCu + H2O2+ H2SO4=== CuSO4 +Γθ_______/p>
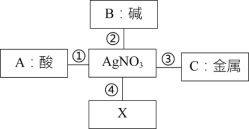
ΓΨΧβΡΩΓΩ2018Ρξ10‘¬16»’ΙΰΕϊ±θ –«χΩΣ ΦΝΥΒΎ“ΜΧλΙ©≈·ΓΘΕ§Χλά¥ΝΥ ς“Ε¬δΝΥΘ§«κΆ§―ßΟ«ΖάΚ°±Θ≈·Θ§ΫΓΩΒ“ϊ ≥Θ§ΕΆΝΕ…μΧεΓΘœ¬ΟφœύΙΊΥΒΖ®’ΐ»ΖΒΡ «
|
|
|
|
AΘ° ςΡΨ…œΆΩΥΔΖά÷ΙΕ≥…ΥΒΡ ·Μ“Ϋ§Θ§÷ς“Σ≥…Ζ÷ «ΧΦΥαΗΤ | BΘ°ΈΗΥαΙΐΕύΒΡ»Υ”ΠΕύ≥‘ΤΜΙϊΓΔιΌΉ” | CΘ°ΕύΚ»Ω…ά÷≤Μάϊ”Ύ…μΧεΫΓΩΒΘ§Ω…ά÷÷–Κ§”–ΧΦΥαΘ§Ήœ…Ϊ ·»ο»ή“ΚΡή ΙΧΦΥα±δΚλ…Ϊ | DΘ°ΤοΉ‘––≥ΒΕΆΝΕ…μΧεΘ§Ή‘––≥Β≥ΒΦήΒΡΫπ τ≤ΡΝœΩ…“‘ «ΟΧΗ÷ΒΡ |
A. A B. B C. C D. D