��Ŀ����
����Ŀ��NaCl ��KNO3�ڲ�ͬ�¶�ʱ���ܽ�����£��ش��������⡣
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
��1��10��ʱ����100 gˮ���ܽ�_________g KNO3ʱ����Һǡ�ôﵽ����״̬��
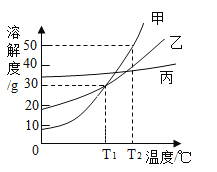
��2����ͼΪNaCl ��KNO3���ܽ�����ߣ����ʾNaCl���ܽ��������_________(����������������)�������¶�t�ķ�Χ������________������ĸ��ţ���
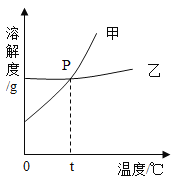
A��10��~20�� B��20��~30��
C��30��~40�� D��40��~50��
��3��10��ʱ���ֱ���100 gˮ�м���35 g NaCl ��KNO3���壬���ȵ�60��ʱ��NaCl��ҺΪ__________����������������������������Һ������ǰ���й�����Һ��˵����ȷ����____________������ĸ��ţ���
A��10��ʱ������Һ�����ʵ�����������ͬ
B��60��ʱ������Һ�����ʵ�����������ͬ
C������ǰ��NaCl��Һ�����ʵ�������������
D������ǰ��KNO3��Һ�����ʵ�������������
���𰸡�20.9 �� B ������ BC
��������
��1���������ܽ�ȱ���֪��10��ʱ����100 gˮ���ܽ�20.9g KNO3ʱ����Һǡ�ôﵽ����״̬��
��2���������ܽ�ȱ���֪������ص��ܽ�����¶�Ӱ��ϴ��Ȼ��Ƶ��ܽ�����¶�Ӱ���С����ͼ�б�ʾNaCl���ܽ���������ң������¶�t�ķ�Χ������20��~30��֮�䣬��ʱ�Ȼ��ƺ�����ص��ܽ���������ཻ�ĵ㣬ѡB��
��3��60��ʱ��NaCl���ܽ����37.3g��10��ʱ���ֱ���100 gˮ�м���35 g NaCl ��KNO3���壬���ȵ�60��ʱ��NaCl��ҺΪ��������Һ��60��ʱ��NaCl���ܽ����37.3g������ص��ܽ����110g����ʱ35 g NaCl ��KNO3����ȫ���ܽ⣬����Һ�����ʵ�����������ͬ��10��ʱ��NaCl���ܽ����35.8g���ʼ���ǰ��NaCl��Һ�����ʵ������������䣬ѡBC��

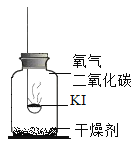
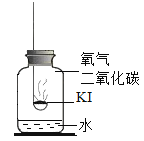
����Ŀ���⻯��(KI)�ǰ�ɫ���壬���治���ᱻ����Ϊ���ʵ�(![]() )�����Ʊ��ʡ�ʵ��С��Ϊ̽���⻯�ر���ԭ�����������»��
)�����Ʊ��ʡ�ʵ��С��Ϊ̽���⻯�ر���ԭ�����������»��
���������ϣ�I.���ڵ⻯�ر��ʵ�ԭ���������ֲ�ͬ�ķ�Ӧ��
�ף�![]() �� �ң�
�� �ң�![]() ��
��
��.![]() ��
��![]() �����ڼ���ߵĻ�ѧ�������ơ�
�����ڼ���ߵĻ�ѧ�������ơ�
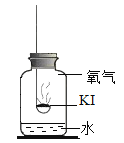
������ʵ�飩ʵ��1��̽����ʵ�ԭ��
ȡ�����⻯�ع��屩¶�ڿ���һ��ʱ�䣬�۲쵽���巺�ơ������ƵĹ����м�������ϡ���ᣬ������ɫ��ζ�����壬ͨ�����ʯ��ˮ�У�����ʯ��ˮ����ǡ�
(1)��ѧС����Ϊ���ݴ��������ܵó�����Ӧ������ɵ⻯�ر��ʵ�ԭ�����Ľ��ۡ�������____
������ʵ�飩ʵ��2��̽�����ʵ�����
�ֱ�ȡ����KI��ȼ�ճ��У��ٷֱ����ʢ�в�ͬ���ʵļ���ƿ�У������������������۲졣
ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
|
|
|
|
����䳱��������� | �������������� | ����䳱���������������� | ����䳱�������� |
����������ۣ�
(2)������ʵ�����֪��KI���ʵ�������________��
��������⣩CO2��������ʲô��
������ʵ�飩
�ֱ�ȡ10mLͬŨ�ȵ�KI��Һ��3֧�Թ���(�Թܱ��1��2��3)�������Թ�2��ͨ��CO2�����Թ�3�еμӼ������ᣬ�ֱ���pH��ֽ�ⶨ��Һ��pH�������Ӻ۲���Һ����ɫ��ʵ�������¼���£�
�Թ���� | 1 | 2 | 3 |
��ҺpH | td style="width:113.7pt; border-style:solid; border-width:0.75pt; padding:3.38pt 5.03pt; vertical-align:middle">pH=6 | pH=4 | |
��Һ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
����������ۣ�(3)��pH��ֽ��ʹ�÷�����_______
��CO2��KI���ʹ����е�������________��
����˼�����ۣ�
(4)̽��KI��������ʱ��ͬѧ���ų��˵�����ϡ�������Ӱ�죬��ԭ����_______
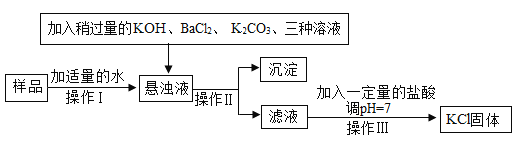
����Ŀ����Ӧ��������Ȳ�ͬ���ܻ�Ӱ������������࣬Ϊ̽��̼��ԭ����ͭ���ɵ��������࣬���������ʵ��(���з�Ӧ����ֽ��У�Ũ������������ˮ����)��
��������⣩��̼��ԭ����ͭ���ɵ�������ʲô��
���������룩������٣�CO ����ڣ�CO2 ����ۣ�CO2��CO
��ʵ����ƣ���װ��ͼ��ͼ
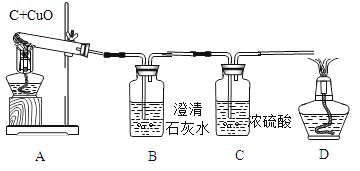
����һ���Ӷ��Թ۲�Ƕ��жϣ�
��ʵ��ʱ��A�г���_________________������
��������ٳ�����B��D����ʵ�������ǣ�
B________________��D_________________��
���������Ӷ�������Ƕ��жϣ�
�ⶨ�����е��ĸ����ݣ�
��Ӧǰ������ | ��Ӧ������� | |
A(�Թ�+����) | m1 | m2 |
B+C(���ƿ+��Һ) | m3 | m4 |
��������ڳ�������(m4-m3)_____(m1-m2)(ѡ����>������<������=��)����ʱA�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��������۳�������8gCuOȫ�����뷴Ӧ����̼�����ʵ���(n)��ȡֵ��Χ��____<n<_____��