��Ŀ����
��V1mL3.65% HCl��Һ��ε���ʢ��V2mLδ֪Ũ�ȵ�
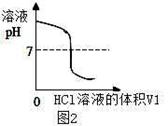
NaOH��Һ���ձ��У�ͼ1������������¼��Һ��pH�仯��ͼ2����
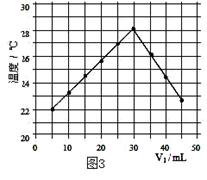
�¶ȱ仯��ͼ3������ʵ����ʼ�ձ��� V1+V2=50.0mL�Ҹ�����Һ�ܶ�
��Ϊ1.0g/mL����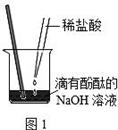
��1��ͼ2��V1="0" mLʱ��ͼ1���ձ�����Һ����ɫ�� ɫ��
��2��ʵ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��ͼ2�е�pH=7ʱ����ʱ�����HCl��Һ�����V1= mL��
��4��ͨ����ʵ��������жϣ�������ȷ���� ��
| A�����и�ʵ��ʱ�����¶�Ϊ22�� |
| B����ʵ�������ѧ�ܿ�ת��Ϊ���� |
| C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ |
| D����Һ�ɼ���ת��Ϊ���ԣ���ת��Ϊ���������Բ��ϼ��� |
��1���죻 ��2��HCl+NaOH�TNaCl+H2O�� ��3��30 �� ��4��B �� ��5��6.0%
���������������1��ͼ2��V1=0mLʱ��ͼ1���ձ�����Һ�Լ��ԣ�����ʹ��̪��죻��2��ʵ���з�����ӦΪ��HCl��NaOH����NaCl��H2O����3����ͼ3�е���Ϣ��֪����Һ�¶����ʱΪ���ǡ����ȫ��Ӧ����Һ������pH=7����ʱ�����HCl��Һ�����V1=30ml����4��A�����и�ʵ��ʱ�����¶�Ϊ22�棬����ȷ����ͼʾ3����Ϣ��֪V1=5mLʱ�¶�Ϊ22�棻B����ʵ�����¶����ߣ������û�ѧ��Ӧ�ų�������������ѧ�ܿ�ת��Ϊ���ܣ�C���û�ѧ��Ӧ������ˮ�������ܴ���ֻҪ����ˮ�ķ�Ӧ�����ȣ�D���÷�Ӧ����Һ�ɼ���ת��Ϊ���ԣ���ת��Ϊ���������Բ�����ǿ��
��5���裬����NaOH��Һ����������Ϊx������������Һ�����ʵ�����Ϊ��30ml��1.0g/ml��3.65%=1.095g
HCl+NaOH�TNaCl+H2O��
36.5 40
1.095g x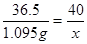 x=1.2g
x=1.2g
ʵ��������NaOH��Һ��������������Ϊ��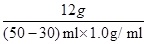 ��100%=6.0%
��100%=6.0%
���㣺�кͷ�Ӧ����Ӧ�ã��й��������������ļ��㣻���ָʾ���������ʣ���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ.

 һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�