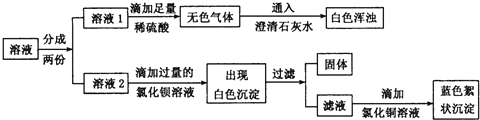
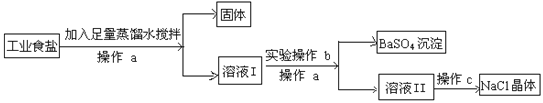
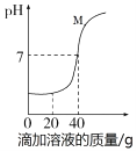
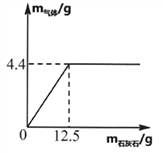
��Ŀ����
����Ŀ��С����ⶨCu��Zn�Ͻ���ͭ������������ʵ����ֻ�ṩ��һƿϡ�������ص�������Ϊ�˲ⶨ�úϽ����ɣ�С����ȡ10�˸úϽ��ĩ���ڷ�ĩ���������μ���ϡ���ᷴӦ��ÿ��һ�����ᣬС����¼���������������ʵ���������£�
��һ�� | �ڶ��� | ������ | |
����������������(ml) | 10 | 10 | 10 |
��������������(g) | 0.08 | 0.08 | 0.04 |
(1)���ϱ����ݷ�����С����10�˺Ͻ��ĩ�ܹ��ռ���___________��������
(2)����úϽ���ͭ����������Ϊ_________��(��ʾ��Cu����ϡ���ᷴӦ��п��ϡ���ᷴӦ�Ļ�ѧ����ʽΪZn+2HCl=ZnCl2+H2��)
���𰸡� 0.2 35%
�����������⿼�����йػ�ѧ����ʽ�ļ��㡣
��1�����ݱ����ṩ�����ݣ�10g�����������0.08g������������ֻ����0.04g��˵������ȫ���μ��˷�Ӧ������������������Ϊ��0.08g+0.08g+0.04g=0.2g��
��2���裺Cu-Zn�Ͻ���п������Ϊx
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
![]() x=6.5g
x=6.5g
Cu-Zn�Ͻ���ͭ����������=![]() ��100%=35%��
��100%=35%��

����Ŀ����ͭ��һ����Ҫ�Ľ���������ͭ��п�ĺϽ𣬿������������������������ճ���Ʒ��Ϊ�˲ⶨ��ͭ��Ʒ����ɣ�ȡ�����Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���£�
��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
ȡ��Ʒ������g�� | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
ȡϡ����������g�� | 30.0 | 60.0 | 90.0 | 120.0 | 150.0 |
��������������g�� | 0.3 | 0.6 | 0.9 | 1.0 | 1.0 |
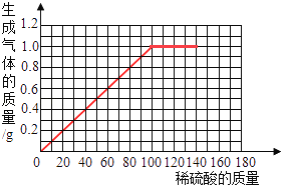
��Ҫ��ش��������⣺
��1������ʵ�����ݷ������ӵ�________�ݿ�ʼ�������Ѿ���Ӧ��ȫ�ˣ�
��2����ʽ����û�ͭ��Ʒ�н���п����������________________����Ҫ��д��������̣�
��3���ڸ���������ֽ�ϣ�����40.0g��Ʒ�м�ϡ�����������������������Ĺ�ϵ����____________��
����Ŀ��ij��ѧ��ȤС��Ϊ�˲ⶨþͭ�Ͻ���þ������������ȡ�˸úϽ���Ʒ2.0g����30ϡ�����6�μ�����Ʒ�С���ַ�Ӧ����ˣ����أ��õ�����������£�
ϡ��������� | ʣ��������� | ϡ��������� | ʣ��������� |
��һ�� 5 g | m | ���Ĵ� 5 g | 0.8 g |
�ڶ��� 5 g | 1.4g | ����� 5 g | 0.6g |
������ 5 g | 1.1g | ������ 5 g | 0.6g |
(1) ������m����ֵΪ________��
(2) ��Ʒ��þ����������Ϊ________��
(3) ������������Һ��������������Ϊ_____________��