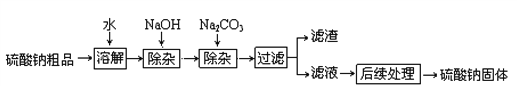
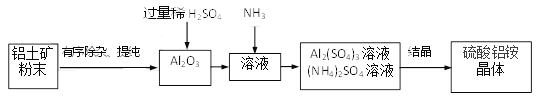
��Ŀ����
����Ŀ����������ȤС��ͬѧ�Ա�¶�ڿ������������ƹ����̽������ش��������⣺
[�������1]�� ���������ƹ�����û�б����أ�
[����ʵ��1]����ͬѧȡ�����������Թ�����������ˮ�ܽ⣬���������� BaCl2 ��Һ���۲쵽_________________��֤���������ƹ����Ѿ����� Na2CO3��
[�������2]��
��γ�ȥ�������ƹ����е����ʣ��õ����������������أ�
[����ʵ��2]����ͬѧ�Ը��������ƹ�������ᴿ�������²������̽���ʵ�飮
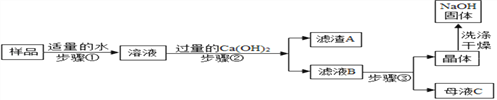
[ʵ�����]��
�Ų���ڷ�Ӧ�Ļ�ѧ����ʽ��____________������ڵIJ����н�����ˣ������������_______��
��֤����������Ѵﵽ Ca(OH)2��Һ������Ŀ�ģ����з����п��е���_________��
a��ȡ������Һ B��������ͨ������̼���壬��Һ�����
b��ȡ������Һ B���������ټ�����ʯ��ˮ���ް�ɫ����
c��ȡ������Һ B���������ټ�����Ũ̼������Һ���а�ɫ����
��Ϊ��ô������������Ʋ�����������ľ������������Ũ����_________�����ˣ� A�������ᾧB�����½ᾧ
�ȱ�ͬѧ��Ϊ��ͬѧ�����е� Ca(OH)2 ��Һ�������Թ����� Ba(OH)2 ��Һ���棬��������Ҫ�ŵ���_______________��
A��������������ˮ����������������ˮ��������������Һ���Լ�����Һ��ˮ���������������Ũ����Ч�ʣ�����Լ��Դ��
B��ֻ�������������ܳ�ȥ����
[ʵ����չ]����βⶨ���õ��ռ���Ʒ���������Ƶ����������أ�
��һƿ���õ��ռ��г�ȡ�� 20g�������ʣ������������Ϊ 19g����ȫ��������ˮ ����� 100g ��Ʒ��Һ����ȡһ�����ʵ������������Ȼ�����Һ����Ʒ��Һ��ϣ���ַ� Ӧ��õ������ʾ�����ݣ�
��Ŀ�ʹ��� | �� 1 �� | �� 2 �� | �� 3 �� | �� 4 �� |
��Ʒ��Һ����(g) | 10 | 20 | 30 | 40 |
�Ȼ�����Һ����(g) | 10 | 15 | 15 | 30 |
��������������(g) | 1.97 | 3.94 | 3.94 | X |
�ɱ��е�_________��ǡ����ȫ��Ӧ��
���������Ʒ���������Ƶ���������Ϊ_____________��(��д����������)
���𰸡� ������ɫ���� Ca(OH)2+Na2CO3===CaCO3��+2NaOH ʹ̼������ȫ��Ӧ ac B A 2��4 44.2%
��������[����ʵ��1]����ͬѧȡ�����������Թ�����������ˮ�ܽ⣬���������� BaCl2 ��Һ���۲쵽������ɫ������֤���������ƹ����Ѿ�����Na2CO3��[ʵ�����]��(1)������У�̼���ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƣ���Ӧ�Ļ�ѧ����ʽ�ǣ�Na2CO3+Ca(OH)2�TCaCO3��+2NaOH������ڵIJ����н��� ���ˣ������������ʹ̼������ȫ��Ӧ��(2)a��ȡ������Һ B��������ͨ������̼���壬��Һ����ǣ�˵������������Һ������b��ȡ������Һ B���������ټ�����ʯ��ˮ���ް�ɫ���ǣ�����˵������������Һ������Ҳ������ǡ����ȫ��Ӧ��c��ȡ������Һ B���������ټ�����Ũ̼������Һ���а�ɫ���ǣ�˵������������Һ������(3)Ϊ��ô������������Ʋ�����������ľ������������Ũ�������½ᾧ��������(4)��ͬѧ��Ϊ��ͬѧ�����е� Ca(OH)2��Һ�������Թ����� Ba(OH)2 ��Һ���棬��������Ҫ�ŵ���������������ˮ����������������ˮ��������������Һ���Լ�����Һ��ˮ���������������Ũ����Ч�ʣ�����Լ��Դ��(5)�ɵ�2�κ͵�3�ο�֪����3������Ʒ��Һ�������ɵ�1�κ͵�2�����ݿ�֪����1���Ȼ�����Һ��������˱��е�2��ǡ����ȫ��Ӧ������Ϊ��4�κ͵�2��������Һ��������ȣ���˵�4��Ҳǡ����ȫ��Ӧ��(6)��Ϊ��2��ǡ����ȫ��Ӧ������Ե�2�����ݽ��м��㣺��20g��Ʒ��Һ��̼��������Ϊx��
BaCl2+Na2CO3�TBaCO3��+2NaCl
106 197
x 3.94g
![]()
x=2.12g��
20g��Ʒ��Һ����Ʒ����Ϊ��20g��![]() ��100%=3.8g��
��100%=3.8g��
��Ʒ���������Ƶ���������Ϊ��![]() ��100%=44.2%��
��100%=44.2%��