��Ŀ����
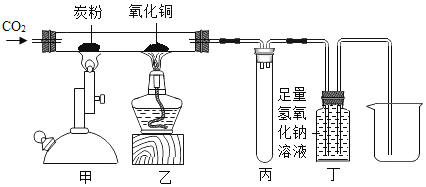
����Ŀ��ijͬѧѧϰ�������Ʊ�֪ʶ�����뵽���ж��ٻƺ���ʢ���ȱ����������Ϻ��֪�ȱ��ǵ���Ҫ�ɷ���̼��ƣ���������ܷ������ȱ��Ǻ�ϡ���ᷴӦ��ȡ������̼��̽��������̼�����ʣ�ͬʱ�ⶨ�ȱ�����̼��Ƶ����������������������һ��̽����
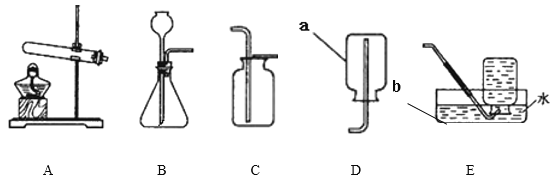
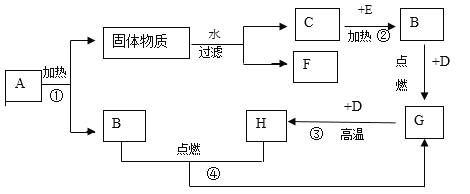
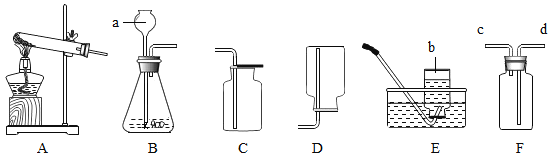
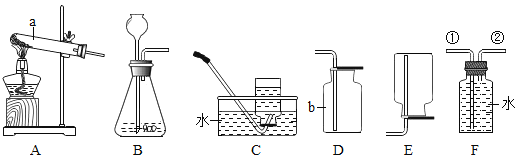
(1)д��ͼ�д��б�����������ƣ�a _______��b ______��
(2)���ȱ��Ǻ�ϡ������ȡ���ռ�������̼һ��ѡ����ͼ�е�װ��Ϊ______(�����)��֤���������Ƕ�����̼�Ļ�ѧ��Ӧ����ʽΪ________��������������ռ����ķ�����_____��
(3)̽�������и�ͬѧ���������ͼ�ķ���װ�ã�ʵ��ʱ���ȱ��Ƿ��ڶ������Ƭ�ϣ�Ȼ��ӳ���©���ڼ�ϡ���ᣬ����ѡװ����ȸ�װ�õ��ŵ���______��
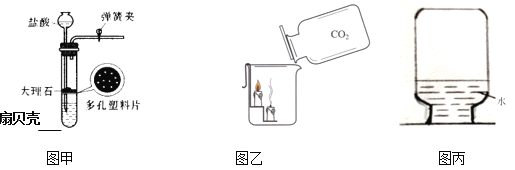
(4)���ռ��Ķ�����̼���嵹��ʢ��ȼ��������ձ���(ͼ��)���۲쵽____����ʵ��ɵó�������̼��������_____����;��____��
(5)��ʢ�ж�����̼�ļ���ƿ�м�����������ˮ����ƿ����(ͼ��)����ͬѧ���ֲ�Ƭ������ƿ������ҧס�����Խ��Ͳ����������ԭ��_____��Ȼ��ȡƿ��ˮ���Թ��У��μ���ɫʯ����Һ��������Թ��м��������Ļ���̿���ã���ʵ���й۲쵽��Һ��ɫ�ı仯�����___��
(6)�ڲⶨ�ȱ�����̼��ƺ���ʱ��ͬѧȷ��ȡ12���ȱ�����Ʒ(�������ʲ�����ˮ�Ҳ����ᷴӦ)�������100������ϡ�����ַ�Ӧ��ʣ�����������Ϊ107.6�ˣ��Լ�����ȱ�����Ʒ��̼��Ƶ���������____(���ݻ�ѧ����ʽ��ʽ���㣬�������һλС��)��
���𰸡�����ƿ ˮ�� BC CO2+Ca(OH)2=CaCO3��+H2O ��ȼ�ŵ�ľ�����ڼ���ƿ�ڣ����Ϩ��˵��������̼���ռ��� ���Կ��Ʒ�Ӧ�ķ�����ֹͣ ȼ�ŵ��������¶�������Ϩ�� ������̼�ܶȱȿ�������ȼ��Ҳ����֧��ȼ�� ��� ������̼������ˮ��ʹ����ƿ��ѹǿ��С ��Һ����ɫ��ɺ�ɫ�ٱ����ɫ 83.3%
��������
��1������a�Ǽ���ƿ������b��ˮ�ۣ��������ƿ��ˮ�ۣ�
(2)���ȱ��Ǻ�ϡ������ȡ���ռ�������̼����Ӧ�����dz��£�������̼�ܶȱȿ���������ˮ��������ȡ���ռ�������̼Ӧѡ�õ�װ��ΪBC��ʵ�����ó���ʯ��ˮ���������̼��֤���������Ƕ�����̼�Ļ�ѧ��Ӧ����ʽΪ��CO2+Ca(OH)2=CaCO3��+H2O��������������ռ����ķ����ǣ���ȼ�ŵ�ľ�����ڼ���ƿ�ڣ����Ϩ��˵��������̼���ռ�����
���BC��CO2+Ca(OH)2=CaCO3��+H2O����ȼ�ŵ�ľ�����ڼ���ƿ�ڣ����Ϩ��˵��������̼���ռ�����
(3)̽�������и�ͬѧ���������ͼ�ķ���װ�ã�ʵ��ʱ���ȱ��Ƿ��ڶ������Ƭ�ϣ�Ȼ��ӳ���©���ڼ�ϡ���ᣬ����ѡװ����ȸ�װ�õ��ŵ��ǿ��Կ��Ʒ�Ӧ�ķ�����ֹͣ��������Կ��Ʒ�Ӧ�ķ�����ֹͣ��
(4)���ռ��Ķ�����̼���嵹��ʢ��ȼ��������ձ���(ͼ��)���۲쵽ȼ�ŵ��������¶�������Ϩ�𣬸�ʵ��ɵó�������̼�������ж�����̼�ܶȱȿ�������ȼ��Ҳ����֧��ȼ�գ���˶�����̼���������
���ȼ�ŵ��������¶�������Ϩ�𣻶�����̼�ܶȱȿ�������ȼ��Ҳ����֧��ȼ�գ����
(5)��ʢ�ж�����̼�ļ���ƿ�м�����������ˮ����ƿ����(ͼ��)����ͬѧ���ֲ�Ƭ������ƿ������ҧס���������������ԭ���ǣ�������̼������ˮ��ʹ����ƿ��ѹǿ��С��Ȼ��ȡƿ��ˮ���Թ��У��μ���ɫʯ����Һ��������Թ��м��������Ļ���̿���ã���ʵ���й۲쵽��Һ��ɫ�ı仯����ǣ���Һ����ɫ��ɺ�ɫ�ٱ����ɫ��
���������̼������ˮ��ʹ����ƿ��ѹǿ��С����Һ����ɫ��ɺ�ɫ�ٱ����ɫ��
��6�����ɶ�����̼������Ϊ��12g+100g-107.6g=4.4g
����ȱ�����Ʒ��̼��Ƶ�����Ϊx

x=10g
���Ը��ȱ�����Ʒ��̼��Ƶ���������Ϊ��![]() ��
��
���83.3%��