��Ŀ����
��10������18��. �����⻯�����⻯�ƣ�CaH2�����⻯�ƣ�NaH����һ����Ҫ�����������ˮ�Ӵ�ʱ���ֱ������·�Ӧ ��
��
CaH2+2H2O=Ca��OH��2+2H2�� NaH+H2O=NaOH+H2��
��1����ij��ѧ��Ӧ��Ҫ����10g��������Ҫ���Ķ��ٿ�NaH��
��2����ɽ�˶�Ա��ɽʱҲ��ͨ�������⻯����ˮ��Ӧ����������ṩ�����������С����Ϊ������CaH2���NaH�������ڵ�ɽ�˶�Ա�������������Ϊʲô��
 ��
��CaH2+2H2O=Ca��OH��2+2H2�� NaH+H2O=NaOH+H2��
��1����ij��ѧ��Ӧ��Ҫ����10g��������Ҫ���Ķ��ٿ�NaH��
��2����ɽ�˶�Ա��ɽʱҲ��ͨ�������⻯����ˮ��Ӧ����������ṩ�����������С����Ϊ������CaH2���NaH�������ڵ�ɽ�˶�Ա�������������Ϊʲô��

��

��ϰ��ϵ�д�
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
�����Ŀ
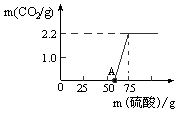


 10CO2 +12H2O���������ɶ�����̼����g��(����������һλС��)
10CO2 +12H2O���������ɶ�����̼����g��(����������һλС��)