��Ŀ����
ijʯ�ҳ���ʯ��ʯ��������ʯ�ң����ⶨʯ��ʯ��ʯ��ʯ�г�̼������������ʲ����ᷴӦҲ������ˮ����̼��Ƶ�������������ش�����
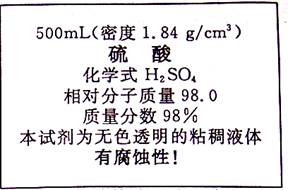
��1������240g��10%ϡ���ᣬ��Ҫ��������40%��ϡ���������� ���������ܶ�1.2g/cm3��
(2)��ʯ��ʯ���飬��ϡ�����ܽ�ķ���ʽΪ
��3����40gʯ��ʯǡ���������Ƶ�ϡ����219g��ȫ��Ӧ�����40gʯ��ʯ��̼��Ƶ�����ΪX�ı���ʽΪ
��4����ʯ��ʯ��̼��ƵĴ���Ϊ
��5����ij����������Ҫ��ʯ��4.2��֣���������95%�������յĹ����й���ʧ5%����ʯ�ң�����Ҫ�������ǻ�ʯ��������
��1������240g��10%ϡ���ᣬ��Ҫ��������40%��ϡ���������� ���������ܶ�1.2g/cm3��
(2)��ʯ��ʯ���飬��ϡ�����ܽ�ķ���ʽΪ
��3����40gʯ��ʯǡ���������Ƶ�ϡ����219g��ȫ��Ӧ�����40gʯ��ʯ��̼��Ƶ�����ΪX�ı���ʽΪ
��4����ʯ��ʯ��̼��ƵĴ���Ϊ
��5����ij����������Ҫ��ʯ��4.2��֣���������95%�������յĹ����й���ʧ5%����ʯ�ң�����Ҫ�������ǻ�ʯ��������
��1��50
(2) CaCO3 + 2HCl= CaCl2 + CO2�� + H2O
��3��100/73=x/21.9g
��4��75%
��5��10���
(2) CaCO3 + 2HCl= CaCl2 + CO2�� + H2O
��3��100/73=x/21.9g
��4��75%
��5��10���
���⿼������Һ��ϡ�ͣ����ݻ�ѧ��Ӧ����ʽ�ļ���ȷ����֪ʶ�㡣
(1)��Һ��ϡ������Ľ���ؼ���ϡ��ǰ�������������䡣����Ҫ��������40%��ϡ��������ΪX������240g��10%=X��1.2g/cm3��40%,��X=50ml
��2���ԣ�3����40gʯ��ʯ��̼��Ƶ�����ΪX
CaCO3 + 2HCl= CaCl2 + CO2�� + H2O
100 73
X 219g10%
100/73=X/219g��10%
X=30g
(4) ��ʯ��ʯ��̼��ƵĴ���Ϊ30g��40g=75%
(5) ����Ҫʯ��ʯ������Ϊx
CaCO3=CaO+CO2
100 56
75%x 4.2��95%��95%
100/56=75%x/4.2��95%��95%
X=10
(1)��Һ��ϡ������Ľ���ؼ���ϡ��ǰ�������������䡣����Ҫ��������40%��ϡ��������ΪX������240g��10%=X��1.2g/cm3��40%,��X=50ml
��2���ԣ�3����40gʯ��ʯ��̼��Ƶ�����ΪX
CaCO3 + 2HCl= CaCl2 + CO2�� + H2O
100 73
X 219g10%
100/73=X/219g��10%
X=30g
(4) ��ʯ��ʯ��̼��ƵĴ���Ϊ30g��40g=75%
(5) ����Ҫʯ��ʯ������Ϊx
CaCO3=CaO+CO2
100 56
75%x 4.2��95%��95%
100/56=75%x/4.2��95%��95%
X=10

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 ��
��