��Ŀ����
��2010��������ɽ��24���ҹ�����������ȫ���һ��ռȫ���ܲ�����30�����ϡ�ij��ȤС�鵽�������������飬ȡ����(����ֻ������̼)����ʵ�顣�����������ȫ��ͬ���ķݸ�����ĩ�зֱ����100gϡH2SO4��Һ����ַ�Ӧ��õ�ʵ���������±���ʾ��
����㣺ϡ������ȫ��Ӧ��ʵ���У���Ӧ����Һ�����ʵ����������Ƕ���?(���������0��1��)
ʵ��l | ʵ��2 | ʵ��3 | ʵ��4 | |
������ĩ���� | 2��84g | 4��26g | 6��23g | 7��5lg |
����H2������ | 0��10g | 0��15g | 0��20g | 0��20g |
����㣺ϡ������ȫ��Ӧ��ʵ���У���Ӧ����Һ�����ʵ����������Ƕ���?(���������0��1��)
�⣺��μӷ�Ӧ����������Ϊx������FeSO4������Ϊy
Fe+ H2SO4 = FeSO4 + H2�� (1��)
56 152 2
X y 0.2g

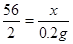 (1��)
(1��)
X ="5.6g " (1��)
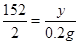 (1��)
(1��)
Y="15.2g " (1��)
����FeSO4��Һ����������������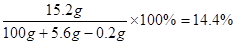 (1��)
(1��)
�𣺷�Ӧ����Һ�����ʵ�����������14.4%
Fe+ H2SO4 = FeSO4 + H2�� (1��)
56 152 2
X y 0.2g

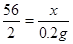 (1��)
(1��)X ="5.6g " (1��)
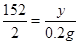 (1��)
(1��)Y="15.2g " (1��)
����FeSO4��Һ����������������
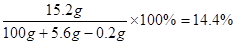 (1��)
(1��)�𣺷�Ӧ����Һ�����ʵ�����������14.4%
��

��ϰ��ϵ�д�
�����Ŀ
 ��
��