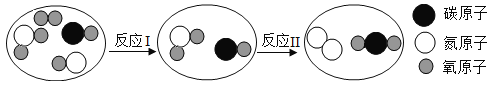
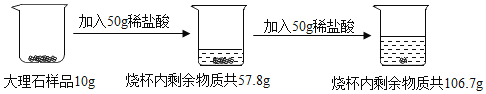
��Ŀ����
����Ŀ��С����������ʴ��������̽��������ʱ����������Ʒ����ֽ���ô�ͷ��̶��������ϣ�Ѹ��������װ����ͼ���۲쵽��Ͳ��ˮ�ص����������뾻�ݻ�Ϊ146mL�Ĺ��ƿ�����¶Ȼָ������£�����Ͳ��ˮ��߶Ȳ��ٱ仯ʱ��������¼��Ͳ��ʼ�����յĶ����Լ�����ʱ�����±���
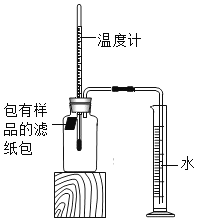
��� | ��Ʒ | ��Ͳ��ʼ ����/mL | ��Ͳ���� ����/mL | ����ʱ�� /min |
�� | 1g���ۡ�0.2g̼��10��ˮ | 100 | 78 | Լ120 |
�� | lg���ۡ�0.2g̼��10��ˮ������NaCl | 100 | 78 | Լ70 |
�� | / | / | / |
��1��ʵ��������˵��NaCl����__���������ӿ�������������������ʴ�����ʣ�
��2��ʵ�鿪ʼ���ƿ���¶�����������˵��������ʴ������__�����������������������������̣�
��3��ʵ�����ֽ�������ɵ���������Ҫ�ɷֵĻ�ѧʽ��__��
��4��ʵ����������̽��̼������ʴ���ʵ�Ӱ�죬��дʵ��������Ʒ��ɣ�__��
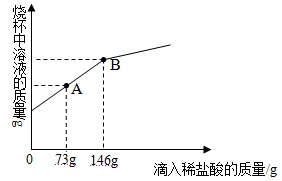
��5��С����Ϊ��װ�û������ڲ��������������ĺ����������������ݼ������������������__%����������3λ��Ч���֣���
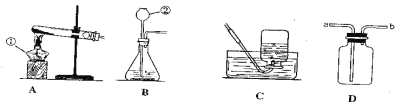
��6��Ϊ��һ����֤��5��С���ʵ�������ֽ��ṩ����Ͳ�����ձ�����������ˮ����ֽ�����ں����ۡ�̼��ˮ��NaCl�����������С�������һ�������������������������ʵ�鷽��______���ɻ���ͼ��ʾ����
���𰸡��ӿ� ���� Fe2O3xH2O 1g���ۡ�10��ˮ 15.1 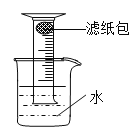
��������
��1��ʵ��ٺ͢�֮��Ψһ�ı���Ϊ�Ƿ���NaCl�����ݺ���NaCl�Ģ���ʵ�飬��Ӧ�����ʱ��϶̣��õ�NaCl�ܼӿ�����ʴ�����ʣ�����ӿ졣
��2��ͨ���¶����ߣ�����ֱ�ӵó�����ʴ�Ĺ����Ƿ��ȵĹ��̣�������ȡ�
��3����������е�������ˮ�����������⣬��Ҫ�ɷ�ΪFe2O3xH2O�����Fe2O3xH2O��
��4����ʵ��ٺ͢���̽��̼������ʴ���ʵ�Ӱ���������Ըö�����Ψһ�ı���ӦΪ�Ƿ���̼����Ϊʵ��ٻ���̼����ʵ��۲���̼��������������Ӧ��ȫ��ͬ������ʵ��۵���Ʒ���Ϊ1g���ۡ�10��ˮ�����1g���ۡ�10��ˮ��
��5����Ϊ��Ͳ��Һ����ٵ������Ϊ���ƿ�������е�ȫ��������������������������������![]() ��100%��15.1%�����15.1%��
��100%��15.1%�����15.1%��
��6���ٻ�����Ͳ�������ձ��У���Ͳ����Һ�����£�����ȷ�����ֽ������Ͳ�е�λ�á��ò�����֧����ֽ���ں�10g���ۡ�0.2g̼��10��ˮ������NaCl�����ڵ��۵���Ͳ�ײ�������Ͳ������ʢ��ˮ���ձ��У��۲���Ͳ��Һ��仯��


 ��У����ϵ�д�
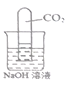
��У����ϵ�д�����Ŀ��ѧϰ�������ƵĻ�ѧ���ʺ�С��������ʵ�飺����ʾ���Ȼ�þ��ҺΪ��ɫ������ʹ��̪��Һ��죩
ʵ�� | ʵ����� | ʵ�������Ԥ������ | ���ۻ���� |
�� |
| ����______________ | ������̼���������Ʒ�����ѧ��Ӧ���÷�Ӧ ����ʽΪ__________ |
�� |
| ��Һ����ɫ��Ϊ��ɫ | ���ۣ�_____________________________ |
�� |
| ����ϡ�����δ�۲쵽�����������̪����Һ���ɫ�� | ��������������δ������ѧ��Ӧ |
�� |
| �а�ɫ�������� | ���ɳ�����ԭ���û�ѧ����ʽ���ͣ� _________________ |
�� | 1.��II��III��IV��֧�Թ��е����ʵ���һ���ྻ���ձ��� | �ձ��г��ְ�ɫ���������ã��ϲ���Һ��ɫ | �ϲ���Һ�����ʵijɷ�Ϊ __________ ����ָʾ���⣩ |
2.ȡ�����ϲ���Һ���Թ��У�����_____��Һ����֪̽����Һ�����ʳɷ� | ���ܳ��ֵ�����Ϊ��________________ |
��1���벹�����~�ߵ����ݡ�
��2��С����ΪС��ʵ��I������Ʋ�����˵��������̼���������Ʒ�����ѧ��Ӧ��Ӧ���ĸĽ���_____
��3��С����ΪС��ʵ��III
��4��С��ͬѧ�����ʵ��III������÷�ָ̪ʾ��������������������ƻ�Ϻ����Һ�м���ijЩ���ʣ�������Ӧ���������жϷ�Ӧ�Ƿ��������з�����ȷ����_________
A ����Na2CO3��Һ��������������ݣ���֤����Ӧ������
B ����CuSO4��Һ�������������ɫ��������֤����Ӧ������
C ������ɫʯ����Һ�������Һ����ɫ����֤����Ӧ������