��Ŀ����
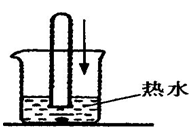
��18�֣�������֣�����ˮ���þ�֮���ڱڳ���һ�㲻����ˮ��ˮ����Ϊ�˽�ˮ���ij��ɷ֣���Ѱ����ϴˮ���ķ�������������ʵ��̽�����̣�������룺
����������Ϻ��֪����Ȼ���е�ˮ�г�����Ca(HCO3)2�ȿ��������ʡ������[�����]��ˮ���������տ�ˮʱCa(HCO3)2����ʱ�����ֽ������Ϊ����֤[�����]�Ƿ���ȷ������������ͼ��ʾʵ�顣
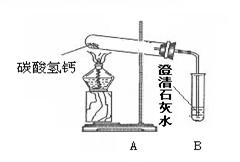
��1������һ��ʱ������Թܿ���ˮ�������B�г��ְ�ɫ���ǣ�д��B�з�����Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��������Թ����а�ɫ������ڣ�������ɫ�������ʢ������ˮ���ձ��У���ֽ��裬�������а�ɫ���塣��ɫ����ijɷ֣�������� �������[�����]:��Ϊ��ɫ���������
a.CaCO3�� b.Ca(OH)2�� c.CaCO3��Ca(OH)2�Ļ����
Ϊ����֤[�����]�������������ȷ�ģ��������������ʵ�鷽����
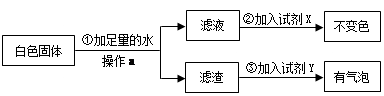
�Լ�X�� ���Լ�Y�� ����[�����]�� ��ѡ�a������b����c������ȷ��
��3���ݴˣ�����Ϊ�����й�ˮ���γ�ԭ���[�����]�� ��ѡ���ȷ��������д���γ�ˮ���Ļ�ѧ����ʽ ��
��4������ͬѧ���ð�ɫ����Ҳ������CaO�������һ���ӷ������Ľ��ۣ�������
��
��5����������̽�������������ˮ������Ҫ�ɷ֣��������ͥ��ϴˮ��ʱ��ѡ�� ��ѡ����ţ���
a.ʳ�� b.ʳ��ˮ c.ˮ
����������Ϻ��֪����Ȼ���е�ˮ�г�����Ca(HCO3)2�ȿ��������ʡ������[�����]��ˮ���������տ�ˮʱCa(HCO3)2����ʱ�����ֽ������Ϊ����֤[�����]�Ƿ���ȷ������������ͼ��ʾʵ�顣

��1������һ��ʱ������Թܿ���ˮ�������B�г��ְ�ɫ���ǣ�д��B�з�����Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��������Թ����а�ɫ������ڣ�������ɫ�������ʢ������ˮ���ձ��У���ֽ��裬�������а�ɫ���塣��ɫ����ijɷ֣�������� �������[�����]:��Ϊ��ɫ���������
a.CaCO3�� b.Ca(OH)2�� c.CaCO3��Ca(OH)2�Ļ����
Ϊ����֤[�����]�������������ȷ�ģ��������������ʵ�鷽����

�Լ�X�� ���Լ�Y�� ����[�����]�� ��ѡ�a������b����c������ȷ��
��3���ݴˣ�����Ϊ�����й�ˮ���γ�ԭ���[�����]�� ��ѡ���ȷ��������д���γ�ˮ���Ļ�ѧ����ʽ ��
��4������ͬѧ���ð�ɫ����Ҳ������CaO�������һ���ӷ������Ľ��ۣ�������
��
��5����������̽�������������ˮ������Ҫ�ɷ֣��������ͥ��ϴˮ��ʱ��ѡ�� ��ѡ����ţ���
a.ʳ�� b.ʳ��ˮ c.ˮ
��ÿС���2�֣���18�֣�
��1��Ca(OH)2 + CO2�� CaCO3�� + H2O
��2�������غ㣻��ɫ��̪��ϡ���c
��3����ȷ��Ca(HCO3)2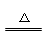 CaCO3�� + H2O + CO2��
CaCO3�� + H2O + CO2��
��4������������ˮ��Ӧ�����������ƣ�ʹ��ɫ�ķ�̪��Һ���ɫ
��5��a
��1��Ca(OH)2 + CO2�� CaCO3�� + H2O
��2�������غ㣻��ɫ��̪��ϡ���c
��3����ȷ��Ca(HCO3)2
 CaCO3�� + H2O + CO2��
CaCO3�� + H2O + CO2����4������������ˮ��Ӧ�����������ƣ�ʹ��ɫ�ķ�̪��Һ���ɫ
��5��a
������һ��̽���⣬̽��̼����Ƶ����ʣ��ѶȱȽϴ�

��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����Ŀ