��Ŀ����
��ͼ��ijС��ͬѧ��֤�����غ㶨��ʵ���Ƭ�Ρ�

(1)��ͼ����ʾ����ƽ������ƽ�⡣Ҫʹ����ƽƽ�⣬Ӧ��ȡ�Ĵ�ʩ ��
(2)С����ͼ����ʾװ�ý���ʵ�顣��Ӧǰ�Ƶ�������װ�ü�����������Ϊ g��Ȼ��ע��ϡ���ᣬ��ַ�Ӧ����֤�������غ㶨�ɵ������� ������ʵ��������������Һ�е����̪��Ŀ���� ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)С��������������Һ��ע��ϡ���ᣬ���������ݲ�������ԭ���� ��

(1)��ͼ����ʾ����ƽ������ƽ�⡣Ҫʹ����ƽƽ�⣬Ӧ��ȡ�Ĵ�ʩ ��
(2)С����ͼ����ʾװ�ý���ʵ�顣��Ӧǰ�Ƶ�������װ�ü�����������Ϊ g��Ȼ��ע��ϡ���ᣬ��ַ�Ӧ����֤�������غ㶨�ɵ������� ������ʵ��������������Һ�е����̪��Ŀ���� ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)С��������������Һ��ע��ϡ���ᣬ���������ݲ�������ԭ���� ��
(1)�Ƚ�������㣬�ٵ���ƽ����ĸ (2)73.2 ��ƽ�Ա���ƽ�⣨����������Ϊ 73.2g��
��֤ϡ����������������Һ�ܷ�����ѧ��Ӧ����ָʾ�кͷ�Ӧ�Ƿ���ȫ��Ӧ��
HCl + NaOH ="==" NaCl + H2O
��3����������������Һ��������ж�����̼��Ӧ��
��֤ϡ����������������Һ�ܷ�����ѧ��Ӧ����ָʾ�кͷ�Ӧ�Ƿ���ȫ��Ӧ��
HCl + NaOH ="==" NaCl + H2O
��3����������������Һ��������ж�����̼��Ӧ��
����:��1����ʹ����ƽʱҪע�����ƽƽ���������ϣ�Ȼ���������㣬�ڵ�����ĸʹ��ƽƽ�⣮
��2������ʱҪ������������������Ŀ̶�ֵ�������Ӧǰ����ƽ��Ȼƽ�����ܹ�֤�������غ㶨�ɣ�
��3�����������������ʼ���ѧ����ʽ����дҲ�ǽ������Ĺؼ����磺�����кͷ�Ӧ������������Һ�������̼�ķ�Ӧ��
�⣺��1������ͼ�п��Կ���������û�й��㣬�������Ե�����ƽ�⣬Ϊ�˵�����ƽ��Ӧ���Ƚ�������㣬�ٵ���ƽ����ĸֱ����ƽƽ�⣮
��2����ͼ�ҿ��Կ������������Ϊ70g������Ŀ̶�Ϊ3.2g����������Ϊ73.2g��Ϊ��˵��ϡ�������������ȷʵ�����˷�Ӧ�����Լ����̪����֤��Ӧ�ķ������������Һ�ĺ�ɫ��ʧ֮����ƽ��Ȼƽ�����ܹ�֤�������غ㶨�ɣ�Ȼ��д���÷�Ӧ�Ļ�ѧ����ʽ��
��3���������Ʒ��ڿ����л�����ж�����̼��Ӧ����̼���ƣ�̼������ϡ���ᷴӦ�����������̼���壮
�ʴ�Ϊ��
��1���Ƚ�������㣬�ٵ���ƽ����ĸ��
��2��73.2����ƽ�Ա���ƽ�⣨����������Ϊ73.2g������֤ϡ����������������Һ�ܷ�����ѧ��Ӧ����ָʾ�кͷ�Ӧ�Ƿ���ȫ��Ӧ����HCl+NaOH�TNaCl+H2O
��3������������Һ������ж�����̼��Ӧ�������ʣ������𰸺������ɣ�
����: ������ʹ����ƽ������ҩƷ��������ע��ʹ�õķ�����ʹ��ǰ�����ȵ���ƽ�⣻����֤������������Ʒ�Ӧʱ��������̪��Һ����ס��ѧ����ʽ��HCl+NaOH�TNaCl+H2O��

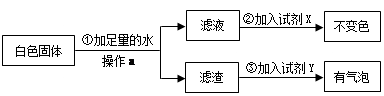
��ϰ��ϵ�д�
�����Ŀ

 �ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________��
�ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________��