��Ŀ����
15���������糧�ͷų��������������NOx����SO2�� CO2���������ɻ��������ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ���1�����������ü������ԭNOx��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ/mol
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2=-1160kJ/mol
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867 kJ/mol��
��2����̼����CO2ת��Ϊ�״���CO2 ��g��+3H2��g���TCH3OH��g��+H2O��g����H3
����ͼ1��25��ʱ�Լ״�ȼ�ϵ�أ��������ҺΪ KOH��Ϊ��Դ������ң�100mL2mol/LAgNO3��Һ���ͱ���100mLCuSO4����Һ��ȼ�ϵ�ظ����ĵ缫��ӦΪCH3OH-6e-+8OH -=CO32-+6 H2O��������������м��� 0.1mol Cu��OH��2��ǡ�ûָ�����Ӧǰ��Ũ�ȣ���������Һ��ˮϡ����200mL����Һ�� pH0��
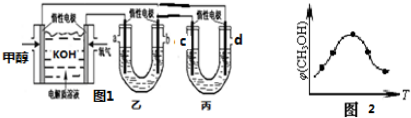
��ȡ��ݵ������CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH���뷴Ӧ�¶�T�Ĺ�ϵ������ͼ2��ʾ��������CO2ת��Ϊ�״��ķ�Ӧ�ġ�H��0�����������������=������
��3������ȼú��������������̼����һ����������������Ӧ��������泥�����ʱ����NH4��2SO4����Һ�е���NaOH��Һ����Һ�����ԣ���������Һ����Ũ�ȴ�С��ϵc��Na+��=c��NH3•H2O���������������������=����
���� ��1����֪����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ•mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2=-1160kJ•mol-1
���ݸ�˹���ɣ�����+�ڣ���$\frac{1}{2}$�ɵã�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g������H=$\frac{1}{2}$����H1+��H2����
��2����ԭ����и����Ϸ���������Ӧ��������CH3OHʧ���ӣ���������������̼�����ˮ��
���м���0.1mol Cu��OH��2ǡ�ûָ�����Ӧǰ��Ũ�ȣ�������������������Ӧ����������˵������������������Ϊ0.1mol����ת�Ƶ���Ϊ0.1mol��4=0.4mol������AgNO3�����ʵ���Ϊ0.1L��2mol/L=0.2mol��������������ȫ�ŵ�ת�Ƶ���Ϊת�Ƶ���Ϊ0.2mol����ˮ����ŵ磬���ݵ���غ��֪�õ�����Ϊ0.2mol����������ϡ�ͺ���ҺpH��
����ͼ��֪������ƽ����¶�Խ�ߣ��գ�CH3OH��ԽС��ƽ�����淴Ӧ���У�
��3�����ݵ���غ㣺c��NH4+��+c��Na+��+c��H+��=2c��SO42-��+c��OH-�����������غ��֪c��NH4+��+c��NH3•H2O��=2c��SO42-���������Һ�����ԣ������жϣ�
��� �⣺��1����֪����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ•mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2=-1160kJ•mol-1
���ݸ�˹���ɣ�����+�ڣ���$\frac{1}{2}$�ɵã�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g������H=$\frac{1}{2}$����H1+��H2��=-867kJ/mol��
��Ӧ�Ȼ�ѧ����ʽΪ��CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867 kJ/mol��
�ʴ�Ϊ��CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867 kJ/mol��
��2���٢�ԭ����и����Ϸ���������Ӧ��������CH3OHʧ���ӣ���������������̼�����ˮ���缫��ӦʽΪ��CH3OH-6e-+8OH -=CO32-+6 H2O��
���м���0.1mol Cu��OH��2ǡ�ûָ�����Ӧǰ��Ũ�ȣ�������������������Ӧ����������˵������������������Ϊ0.1mol����ת�Ƶ���Ϊ0.1mol��4=0.4mol������AgNO3�����ʵ���Ϊ0.1L��2mol/L=0.2mol��������������ȫ�ŵ�ת�Ƶ���Ϊת�Ƶ���Ϊ0.2mol����ˮ����ŵ磬���ݵ���غ��֪�õ�����Ϊ0.2mol��ϡ�ͺ���Һ��������Ũ��Ϊ$\frac{0.2mol}{0.2L}$=1mol/L����ϡ�ͺ���ҺpH=-lg1=0��
�ʴ�Ϊ��CH3OH-6e-+8OH -=CO32-+6 H2O��0��
����ͼ��֪��ߵ㷴Ӧ����ƽ�⣬��ƽ����¶�Խ�ߣ��գ�CH3OH��ԽС��ƽ�����淴Ӧ���У������¶�ƽ�����ȷ�����У��淴ӦΪ���ȷ�Ӧ��������ӦΪ���ȷ�Ӧ������H3��0��
�ʴ�Ϊ������
��3�����ݵ���غ㣺c��NH4+��+c��Na+��+c��H+��=2c��SO42-��+c��OH-������Һ�����ԣ���c��H+��=c��OH-������c��NH4+��+c��Na+��=2c��SO42-�����������غ��֪c��NH4+��+c��NH3•H2O��=2c��SO42-���������ɵ�c��Na+��=c��NH3•H2O����
�ʴ�Ϊ��=��
���� ���⿼���Ȼ�ѧ����ʽ���绯ѧ�����㡢��ѧƽ��Ӱ�����ء�����Ũ�ȴ�С�ȽϽ�ȣ�������ѧ�������������������������Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

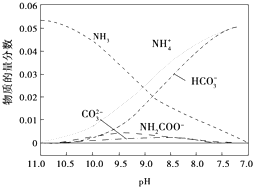
| A�� | ��pH=9.0ʱ��c��NH4+����c��HCO3-����c��NH2COO-����c��CO32-�� | |
| B�� | ��ͬpH����Һ�д��ڹ�ϵ��c��NH4+��+c��H+���T2c��CO32-��+c��HCO3-��+c��NH2COO-��+c��OH-�� | |
| C�� | ����ҺpH���Ͻ��͵Ĺ����У��к�NH2COO-���м�������� | |
| D�� | ����CO2��ͨ�룬$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$�������� |
| A�� | c��Na+��=c��HA-��+2c��A2-��+c��OH-�� | |
| B�� | c��H2A��+c��HA-��+c��A2-��=0.1 mol•L-1 | |
| C�� | ��������Һϡ����0.01mol/L��c��H+��•c��OH-�� ���� | |
| D�� | c ��A2-��+c ��OH-��=c ��H+��+c ��H2A�� |
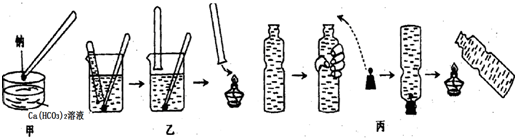

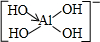 �����Ʋ�1mol NH4BF4��������泥��к���2NA����λ����
�����Ʋ�1mol NH4BF4��������泥��к���2NA����λ����
 ��
��