��Ŀ����
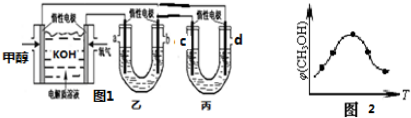
5�����Ʒ�Ӧ�����ɸı仯ѧ��Ӧ���ʣ���1��ʹ�ô����Ǹı仯ѧ��Ӧ���ʵ�һ�ַ���������H2O2��MnO2�����·ֽ⣬��д���÷�Ӧ�Ļ�ѧ����ʽ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2�����о�����Fe3+��Cu2+��H2O2�ķֽ�Ҳ���д����ã�
ij�о�С��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч�������������ʵ�飬�뽫���ǵ�ʵ�鷽������������
ʵ��Ŀ�ģ��Ƚ�Fe3+��Cu2+�Էֽ�H2O2�Ĵ�Ч��
ʵ���Լ���5%H2O2��Һ��0.1mol/LFeCl3��Һ��0.1mol/LCuSO4��Һ
ʵ���������Թܡ���ͷ�ι�
ʵ�鲽�裺ȡ��֧�Թܸ�����1mL5%H2O2��Һ���ֱ�μ�����0.1mol/LFeCl3��Һ��0.1mol/LCuSO4��Һ��
��С���ͬѧ��ͨ���۲�۲����ݲ����Ŀ����ԱȽϳ�Cu2+��Fe3+�Ĵ�Ч����
���������ۣ���ͬѧ�����FeCl3��ΪFe2��SO4��3������������Ϊ���������ǿ�����������ͬ�������ų������ӵĸ��ţ�
��3��Ӱ�컯ѧ��Ӧ���ʵ����س������⣬�����¶ȣ�Ũ�ȵȣ������һ�ּ��ɣ�������һ������������ʵ������˵��������ʳ���ױ��ʣ�����ӿ������ʴ����
���� ��1�����ݹ����������ֽ����������д��
��2��������Ŀ��Ϣ��Ѱ��������Ϣ������H2O2�ڶ������������·����ֽⷴӦ��ʵ���������������ͬ����ĶԱ��������ش𣻸���H2O2��Һ�ֽ��������Ե�ʵ��������ֵĿ������жϣ��öԱ�������ѡ����Լ��о����ܶ���й�ͬ�����ӣ�����ʵ��������˵������
��3������Ӱ�컯ѧ��Ӧ���ʵ����ؽ������ʵ�����ش�
��� �⣺��1��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2H2O$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�����ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2��ij�о�С��Ϊ�Ƚ�Fe3+��Cu2+�Էֽ�H2O2�Ĵ�Ч������˸�ʵ�飬����ʵ��Ŀ���DZȽ�Fe3+��Cu2+�Էֽ�H2O2�Ĵ�Ч����H2O2��Һ�ֽ��������Ե�ʵ�����������������ɣ���С���ͬѧ��ͨ���۲����ݲ����Ŀ������ȽϷ�Ӧ���ʣ���FeCl3��ΪFe2��SO4��3�������ͺ������õ�CuSO4��Һ������ͬ�������ӣ������ų������ӵĸ��ţ�
�ʴ�Ϊ���Ƚ�Fe3+��Cu2+�Էֽ�H2O2�Ĵ�Ч�����۲����ݲ����Ŀ�����������������ͬ�������ų������ӵĸ��ţ�
��3��Ӱ�컯ѧ��Ӧ���ʵ����ػ����¶ȣ�Ũ�ȵȣ�����������ʳ���ױ��ʣ�����ӿ������ʴ����
�ʴ�Ϊ���¶ȣ�Ũ�ȵȣ�������ʳ���ױ��ʣ�����ӿ������ʴ����
���� ������һ��ʵ��̽���⣬������Ӱ���������ֽ����ʵ����أ����������ʵ�����һ����ѧ���������ϵ���Ŀ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 2-��-3-�һ����� | B�� | 3��4��4-�������� | ||
| C�� | 2-��-4-�һ����� | D�� | 1��2-�������� |
| Ԫ�� | H | Li | Be | B | C | N | O | F |
| �縺�� | 2.1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 | 3.0 | 0.8 |
��1��Ԥ�����ڱ��е縺������Ԫ��ӦΪF�����Ƹ�Ԫ�صĵ縺�Ե�ȡֵ��Χ��0.8��X��1.2��
��2�����ݱ��е��������ݷ�����ͬ�����ڵIJ�ͬԪ��X��ֵ�仯�Ĺ��������϶��µ縺�Խ��ͣ�
����Ԫ�ص縺��X�Ĵ�С��Ԫ�ؽ����ԡ��ǽ�����֮��Ĺ�ϵ�ǽ�����Խǿ�縺��Խ������Խǿ�縺��ԽС��
��3��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3���γɵĻ�ѧ��������Ϊ���ۼ�����������AlCl3�����縺��֮��Ϊ1.5��BrԪ�صĵ縺��С��ClԪ�ص縺�ԣ�AlBr3�����縺��֮��С��1.5��

| A�� | ͼ1��ʵ�����Ʊ�Fe��OH��2 | |
| B�� | ͼ2����NaCl��I2�Ļ�����л���I2 | |
| C�� | ͼ3���ռ�NO���� | |
| D�� | ͼ4������������ˮ������Ӧ���������� |
��13Al3+ ��
 ��
�� ��F- ���к����������ͬ���ǣ�������
��F- ���к����������ͬ���ǣ�������| A�� | �٢� | B�� | �ڢۢ� | C�� | �ڢ� | D�� | �٢ۢ� |
| A�� |  ����-OH ����-OH | B�� |  ����-CHO ����-CHO | ||
| C�� |  ȩ��-CHO ȩ��-CHO | D�� | CH3-O-CH3 ����  |
| A�� | Һ̬ˮ���ܶȴ��ڱ����ܶ� | B�� | �ⵥ�ʺɱ��������� | ||
| C�� | �����������Բ��������������� | D�� | �������������Ȼ�̼ |
 ��A��B��C��D��E����Ԫ�أ��������Ϣ���£�
��A��B��C��D��E����Ԫ�أ��������Ϣ���£�| Ԫ�� | �����Ϣ |
| A | AԪ�ص�һ��ԭ��û�����ӣ�ֻ��һ������ |
| B | B�ǵ縺������Ԫ�� |
| C | C�Ļ�̬ԭ��2p���������δ�ɶԵ��� |
| D | DΪ����Ԫ�أ�����Eͬ���ڣ�����������������˶�״̬��ͬ�ĵ��� |
| E | E���γ�ש��ɫ����ɫ����E2O��EO���������� |
��1��д��EԪ��ԭ�ӻ�̬ʱM��ĵ����Ų�ʽ3s23p63d10
��2��CԪ�ص��ʷ����к��Цĺͦм��ļ���֮��Ϊ1��2��
��3��������ˮ�е��ܽ��C7H15OH���Ҵ��͵�ԭ���ǣ��Ҵ��е��ǻ���ˮ���ǻ��ṹ���������ܽ�ȴ�C7H15OH����������ˮ���ǻ��ṹ���Ƴ̶�С�����ܽ��С��
��4��A��C��E����Ԫ�ؿ��γɣ�E��CA3��42+�����ӣ����д��ڵĻ�ѧ�������Т٢ۣ�����ţ���
����λ�� �ڽ����� �ۼ��Թ��ۼ� �ܷǼ��Թ��ۼ� �����Ӽ� �����
�� E��CA3��42+���жԳƵĿռ乹�ͣ��ҵ� E��CA3��42+�е�����CA3���ӱ�����Clȡ��ʱ���ܵõ����ֲ�ͬ�ṹ�IJ���� E��CA3��42+�Ŀռ乹��Ϊa������ţ���
a��ƽ��������b���������� c�������� d��V��
��5�����������ǻ���ɫ��״�ᾧ����ṹ��ͼ��ʾ��
������������Pԭ�Ӳ�ȡsp3�ӻ�����PO3-��Ϊ�ȵ�����Ļ�������ӵĻ�ѧʽΪSO3����NA��ʾ�����ӵ���������ֵ��0.1mol�����������к��еŵ��Ӷ���ΪNA��