��Ŀ����
5��ijѧ��Ϊ�ⶨ��ʯ�����ɣ�CaSO4•xH2O�������ⶨxֵ��������ʵ�飺����ʯ����������м��ȣ�����ǰ����������������ʵ��������ӣ�����ʱ�䲻���ӳ��������������ǰ������������������| ʵ��˳�� | ����ǰ/g | ���Ⱥ�/g |
| 1 | 3.44 | 3.26 |
| 2 | 3.44 | 3.12 |
| 3 | 3.44 | 2.90 |
| 4 | 3.44 | 2.90 |
| 5 | 3.44 | 2.80 |
| 6 | 3.44 | 2.78 |
| 7 | 3.44 | 2.72 |
��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽΪCaSO4•2H2O
��2��CaSO4��Ħ��������136/mol
��3����3��ʵ��ʱCaSO4•xH2O�ֽ�õ�0.01mol��ʯ�࣬������ʯ��Ļ�ѧʽ��aCaSO4•bH2O����a��b��������������a=2��b=1��
���� ��1��ʯ����ȷֽ���ٵ���������ˮ����������2��ʵ��7����֪3.44gCaSO4•xH2O��ȫ�ֽ�õ���ˮCaSO42.72g��������0.72g�����ݲ������⣻
��2��Ħ����������ֵ���������������ֵ����ȣ���λ��g/mol��
��3��0.01mol��ʯ��aCaSO4•bH2O��CaSO4��0.01amol��ˮ��0.01bmol�������CaSO4•2H2O�����ʵ����ͷֽ�õ�ˮ�����ʵ���������ԭ���غ����a��b��ֵ��
��� �⣺��1��ʯ����ȷֽ���ٵ���������ˮ����������ʵ������֪3.44gCaSO4•xH2O��ȫ�ֽ�õ���ˮCaSO42.72g��������0.72g����
CaSO4•xH2O�TCaSO4+xH2O
136 18x
2.72 0.72
��136��2.72=18x��0.72�����x=2������ʯ��Ļ�ѧʽΪCaSO4•2H2O���ʴ�Ϊ��CaSO4•2H2O��
��2��Ħ����������ֵ���������������ֵ����ȣ���λ��g/mol��CaSO4�����������136������Ħ������Ϊ136/mol���ʴ�Ϊ��136/mol��
��3��0.01mol��ʯ��aCaSO4•bH2O��CaSO4��0.01amol��ˮ��0.01bmol��3.44gCaSO4•2H2O�����ʵ���Ϊ3.44g��172g/mol=0.02mol����0.01a=0.02��a=2��0.02molCaSO4•2H2O�нᾧˮ��0.04mol����3��ʵ����Ⱥ��������2.90g��˵����3.44g-2.90g=0.54gˮ���ɣ������ʵ���Ϊ0.54g��18g/mol=0.03mol������ʯ��aCaSO4•bH2O�нᾧˮ�����ʵ���Ϊ0.01mol����0.01b=0.01����b=1���ʴ�Ϊ��2��1��
���� ���⿼�����ʵ���ɣ����ò�����ͨ����������仯��ã�ע������������ݣ��ҵ����۲�����ʵ�ʲ����ǽ���Ĺؼ����Ѷ��жȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �ò�����պȡ��ˮ����pH��ֽ�ϣ��������ɫ����ɫ�����ˮ��pHֵ | |
| B�� | ������������Һ�е������ӣ�ȡ�����μ�1��2����������Һ������Һ����ǣ�����Һ�к���Cl- | |
| C�� | �ɲ�����ȴ�ᾧ�ķ����ӻ���KNO3��NaCl��Һ���ᴿNaCl | |
| D�� | ������ʵ��һ�������������ƿ��û�ӷ�ʯ��Ӧֹͣ���ȣ�����ȴ�ӷ�ʯ |
| A�� | H2 | B�� | HCl | C�� | KCl | D�� | CO2 |

| A�� | CH3COOH HCl�� CH3COONa | B�� | H2SO4�� ��NH4��2SO4��CH3COONa | ||
| C�� | ��NH4��2SO4�� CH3COOH�� NaOH | D�� | ��NH4��2SO4��NH4Cl��CH3COONa |
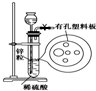 ��ͼΪʵ�����������ļ���װ�ã����ڼ�ϡ����ʱ����п����ϡ����û�нӴ���ϡ������ˣ�Ϊʹ�÷�Ӧ˳�����У����Դӳ���©���м�����Լ��ǣ�������
��ͼΪʵ�����������ļ���װ�ã����ڼ�ϡ����ʱ����п����ϡ����û�нӴ���ϡ������ˣ�Ϊʹ�÷�Ӧ˳�����У����Դӳ���©���м�����Լ��ǣ���������ʳ��ˮ
��KNO3��Һ
������ϡ����ͭ��Һ
��Na2CO3��Һ
��CCl4
��Ũ��ˮ��
| A�� | �٢ۢ� | B�� | �٢ڢ� | C�� | �ܢݢ� | D�� | �٢ڢۢ� |
| A�� | FeS��CuS | B�� | FeS | C�� | CuS | D�� | Al2S3 |
| A�� | �ȳ��ֳ�������ȫ���ܽ� | |
| B�� | ��Al3+��SO42-ȫ���������ó�������� | |
| C�� | ���ֻ��BaSO4���� | |
| D�� | �������0.1mol Al��OH��3��0.2molBaSO4 |