��Ŀ����
4��ʵ������һƿ������Ʒ�����ܺ���NH4HC03��NH4Cl��NH4N03�е�һ�ֻ���֣�ͨ������ʵ�����ɷ֣�������Ʒ23.90g������100mL2.0mol•L-1�����ַ�Ӧ����Һ�����ԣ��ҷų��������ڱ�״����Ϊ2.24L������������Ӧ�������Һ�м�������AgN03��Һ�����ٲ������������ó���Ϊ28.7g����������ʵ����ʵ�ó��Ľ�����ȷ���ǣ�������| A�� | ����Ʒ��һ������NH4HC03��NH4Cl | |
| B�� | ����Ʒ�к�NԪ�ص���������Ϊ17.57% | |
| C�� | ����Ʒ���������ռӦ��������0.4mol NaOH | |
| D�� | �����������ݿ���ȷ��ԭ��Ʒ�к���5.3g NH4N03 |
���� ������Ʒ23.90g������100mL2.0mol•L-1�����ַ�Ӧ����Һ�����ԣ��ҷų��������ڱ�״����Ϊ2.24L��������Ϊ������̼����һ������NH4HC03����n��NH4HC03��=n��C02��=$\frac{2��24L}{22.4L/mol}$=0.1mol��������������ʵ���Ϊn��HCl��=0.1L��2mol/L=0.2mol��
��������Ӧ�������Һ�м�������AgN03��Һ�����ٲ������������ó���Ϊ28.7g��ӦΪAgCl��������n��AgCl��=$\frac{28.7g}{143.5g/mol}$=0.2mol��˵������NH4Cl�������Ʒ�����ж��Ƿ���NH4N03���Դ˽����⣮
��� �⣺������Ʒ23.90g������100mL2.0mol•L-1�����ַ�Ӧ����Һ�����ԣ��ҷų��������ڱ�״����Ϊ2.24L��������Ϊ������̼����һ������NH4HC03����n��NH4HC03��=n��C02��=$\frac{2��24L}{22.4L/mol}$=0.1mol��
������������ʵ���Ϊn��HCl��=0.1L��2mol/L=0.2mol��
��������Ӧ�������Һ�м�������AgN03��Һ�����ٲ������������ó���Ϊ28.7g��ӦΪAgCl��������n��AgCl��=$\frac{28.7g}{143.5g/mol}$=0.2mol��
���ʵ���������������ʵ�������˵������NH4Cl��
�����Ʒ�����ж��Ƿ���NH4N03��
��n��NH4HC03��=0.1mol��79g/mol=7.9g��23.90g����һ������NH4N03��������Ϊ23.90g-7.9g=16g��n��NH4N03��=$\frac{16g}{80g/mol}$=0.2mol��
A�������Ϸ�����֪һ������NH4Cl����A����
B����Ʒ����0.1molNH4HC03��0.2molNH4N03����m��N��=0.5mol��14g/mol=7g��NԪ�ص���������Ϊ$\frac{7g}{23.90g}��100%$=29.28%����B����
C����Ʒ����0.1molNH4HC03��0.2molNH4N03�����������Ʒ�Ӧ�ķ���ʽ�ֱ�ΪNH4HC03+2NaOH=NH3•H2O+Na2C03+H2O��NH4N03+NaOH=NH3•H2O+NaN03���ɷ���ʽ��֪����0.4mol NaOH����C��ȷ��
D�������Ϸ�����֪ԭ��Ʒ�к���16g NH4N03����D����
��ѡC��
���� ���⿼�����ʵļ���ͼ���Ϊ��Ƶ���㣬������ѧ���ķ��������ͼ��������Ŀ��飬ע��������ʵ����ʣ���������غ�����⣬�Ѷ��еȣ�

| A�� | Mg��Al | B�� | S��P | C�� | K��Cs | D�� | Br��Cl |
| A�� | ���º��ݣ���ƽ����ٳ���N2O4���壬NO2�ٷֺ�����С | |
| B�� | ����ѹǿ��ƽ��������Ӧ�����ƶ������������ɫ��dz | |
| C�� | ��ƽ��������¶ȣ���H��С | |
| D�� | 2v����NO2���Tv����N2O4��˵����Ӧ�ﵽ��ѧƽ��״̬ |
| A�� | ��ȥCO2�л��е�CO��ͨ��O2��ȼ | |
| B�� | ��ȥͭ�������ͭ�̡�Cu2��OH��2CO3������������ݣ�������ˮ��ϴ | |
| C�� | ��ȥFeCl3��Һ�е�FeCl2���ʣ������������ۣ��ٹ��˼��� | |
| D�� | ����ȥ�����е�MgCl2��CaCl2�����������ʣ��ɼ����ҩƷ˳��NaOH-Na2CO3-BaCl2-���� |
| A�� | ����ϡ���ᷴӦ 2Fe+6H+�T2Fe3++3H2�� | |
| B�� | �����������������ᷴӦ Al��OH��3+3H+�TAl3++3H2O | |
| C�� | �ƺ���ˮ��Ӧ Na+2H2O�TNa++H2��+OH- | |
| D�� | ͭƬ��ϡ���ᷴӦ Cu+NO3-+4H+�TCu2++NO��+2H2O |
| A�� | 1mol�Ҵ����������������Ʒ�Ӧ������0.5molH2����֤���Ҵ�������һ��Hԭ���������Hԭ�Ӳ�ͬ | |
| B�� | ���з�Ӧ��֤�������ܼ���Ӱ�죬�ױ��ױ����Ը����������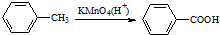 | |
| C�� | ���з�Ӧ��֤�������ǻ��Ա�����Ӱ�죬���±��ӵ�ȡ���ȱ�����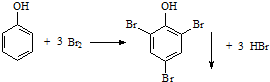 | |
| D�� | ������ʹ������Ȼ�̼��Һ��ɫ����˵����������û������ϩ���������Ƶ�̼̼˫�� |
| A�� | ����������Ǻ��������ֱ���ȫȼ�շų������� | |
| B�� | �к������pH����ȵĴ�����Һ�����������ĵ�NaOH�����ʵ��� | |
| C�� | 2H2��g��+O2��g���T2H2O��l����H1 2H2��g��+O2��g���T2H2O��g����H2��������Ӧ�ȡ�H�Ĵ�С | |
| D�� | ��ͬ�¶ȡ���ͬŨ�ȵ�NH4Cl��NH4HSO4��Һ��c��NH4+�� |
 M��N��O��P��Q��Ԫ�����ڱ���ԭ���������ε�����ǰ������Ԫ�أ�Mԭ������������Ϊ�ڲ��������3����N����ɫ��Ӧ�ʻ�ɫ��O���⻯����һ��ǿ�ᣬ��Ũ��Һ����M��Q�Ļ����ﷴӦ����O�ĵ��ʣ�P��һ�ֽ���Ԫ�أ����̬ԭ������6��δ�ɶԵ��ӣ���ش��������⣺
M��N��O��P��Q��Ԫ�����ڱ���ԭ���������ε�����ǰ������Ԫ�أ�Mԭ������������Ϊ�ڲ��������3����N����ɫ��Ӧ�ʻ�ɫ��O���⻯����һ��ǿ�ᣬ��Ũ��Һ����M��Q�Ļ����ﷴӦ����O�ĵ��ʣ�P��һ�ֽ���Ԫ�أ����̬ԭ������6��δ�ɶԵ��ӣ���ش��������⣺ �ɵ��»��߳���ͷʹ�����ĵȲ�״���農��ע��С�մ�NaHCO3����Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⣮д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
�ɵ��»��߳���ͷʹ�����ĵȲ�״���農��ע��С�մ�NaHCO3����Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⣮д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ�� ��
��