��Ŀ����
13�����ʵ������ܴﵽʵ��Ŀ���ǣ�������| ʵ����� | ʵ��Ŀ�� | |
| A | ��±�������뵽��NaOH��Һ��һ��ʱ���ȡ�ϲ�Һ�壬����AgNO3Һ�������� | ֤��±�����к���±Ԫ�� |
| B | ��ʯ��ˮ��Ӧ�Ƶõ�����ֱ��ͨ������KMnO4��Һ | ֤���Ƶõ������Ƿ�Ϊ��Ȳ |
| C | �����������������ˮ�⣬������������ͭ����Һ���뵽ˮ������Һ�� | �������ˮ��IJ��������� |
| D | ���еμӴ��ᣬ��������������ͨ�뱥��̼��������Һ��ͨ�뱽����Ũ��Һ | ֤�����ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A������±����ˮ����Ӧ���кͼ�ټ�����������±���ӣ�
B����ʯ��ˮ��Ӧ��������Ȳ�����ܻ��л�ԭ���������⣬�����˼��飻
C��Ӧ���ڼ���������ͭ��Һǰ�����������к�ϡ���ᣬ����ʵ���ʧ�ܣ�
D�������ӷ������ñ���̼��������Һ��ȥ���ᣬȻ���ٽ�����ͨ�뱽����Һ��
��� �⣺A��±�����м���NaOH��Һ��ˮ�����±������Ӧ��������Һ�У���Ӧ�ȼ����������ԣ��ټ���AgNO3��Һ���飬��A����
B����ʯ��ˮ��Ӧ��������Ȳ�����ܻ��л�ԭ���������⣬��Ȳ��������ܱ�����KMnO4��Һ���������ܼ����Ƶõ������Ƿ�Ϊ��Ȳ����B����
C������������������ˮ�����������ǣ���������������ͭ��Һ�ķ�ӦӦ�ڼ��������½��У���C����
D�����еμӴ��ᣬ��Ӧ���ɶ�����̼���壬���ڴ����ӷ����ʽ�������������ͨ�뱥��̼��������Һ��ȥ���ᣬȻ����ͨ�뱽����Ũ��Һ�����ӱ���ǣ���֤�����ԣ����̼����ӣ���ʵ���ܹ��ﵽʵ��Ŀ�ģ���D��ȷ��
��ѡD��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ��漰�л���Ľṹ�����ʡ�����ǿ���Ƚϡ����ʼ����֪ʶ���������ʵ����ʡ���ѧ��Ӧԭ����ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飮

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д� �ҹ���ѧ�ҹ�����һ��˫������ȼ�ϵ�أ��Ա��ӣ�C6H6O��Ϊȼ�ϣ�ͬʱ�������Է�ˮ�е������Σ�����˵����ȷ���ǣ���������
�ҹ���ѧ�ҹ�����һ��˫������ȼ�ϵ�أ��Ա��ӣ�C6H6O��Ϊȼ�ϣ�ͬʱ�������Է�ˮ�е������Σ�����˵����ȷ���ǣ���������| A�� | aΪ���� | |
| B�� | ���ҳز���0.672L���壨����£�����ת�Ƶ���0.15mol | |
| C�� | ��ص缫��ӦʽΪC6H6O+11H2O-28e-�T6CO2��+28H+ | |
| D�� | ������ĵı������ҳ����ĵ�NO3-�����ʵ���֮��Ϊ28��5 |
| A�� | ���ӻ�����AB | B�� | ���ӻ�����B2A3 | C�� | ���ӻ�����B��AO3��2 | D�� | ���ӻ�����A2BO3 |
| A�� | �������ӵĵ���ʽ�� | B�� | CS2���ӵĽṹʽ��S�TC�TS | ||
| C�� | S2-�Ľṹʾ��ͼ�� | D�� | CH4�ķ���ģ��ʾ��ͼΪ�� |
| A�� | BF3 | B�� | P4 | C�� | CO2 | D�� | NH3 |

| A�� | һ������Al��������Ϊ2.7g | |
| B�� | һ��������FeCl2����������AlCl3 | |
| C�� | ��������0.05molMgCl2 | |
| D�� | һ�����У�NH4��2SO4��MgCl2�������ʵ������ |
| �� | |||||||
| �� | �� | �� | |||||
| �� | �� | �� | �� | �� | |||
��2���������Ԫ���а뾶���ij���������S2-��
��3����������������Ӧˮ����ļ�����ǿ�����ʵĵ���ʽΪ
 ��������ǿ�����ʵĻ�ѧʽ��HClO4����̬�⻯�����ȶ������ʵĻ�ѧʽ��HF��
��������ǿ�����ʵĻ�ѧʽ��HClO4����̬�⻯�����ȶ������ʵĻ�ѧʽ��HF����4��Ԫ�طǽ�����ǿ���Ƚ��кܶ�������Тۺ͢�ķǽ�����ǿ����̽�������в����е���A������ţ�
A���Ƚ������⻯����۷е�ߵ� B���Ƚ��⻯����ȶ���
C��ͨ��������Ӧ���Ƚϵõ������� D��ͨ���û���Ӧ
��5���ɢٺ͢�����Ԫ����ɵĻ����ͬ�������������ܶ��൱����д���÷��ӵĵ���ʽ
 ��
����6���������Ԫ�صĵ��ʷ�Ӧ����1mol�ߵ���ۻ�����ָ������£�����687kJ����֪�û�������ۡ��е�ֱ�Ϊ-69���58�棬д���÷�Ӧ���Ȼ�ѧ����ʽSi��s��+2Cl2��g��=SiCl4��l����H=-687kJ/mol��
| A�� | ������1L0.1mol•L-1NH4NO3��Һ�еĵ�ԭ����Ϊ0.2NA | |
| B�� | ��1molH2SO4��Ũ�����������п��ȫ��Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| C�� | A��B��C��DΪ������Ԫ�ع��ɵ��������ʣ�����������ת����ϵ����DΪǿ����ʣ�����������ʿ���ʡ�ԣ���A$\stackrel{O_{2}}{��}$B$\stackrel{O_{2}}{��}$C$\stackrel{H_{2}O}{��}$D����A�ǹ��ۻ����0.1molA�����к��е�����������ΪNA | |
| D�� | ������CO��ԭ������õ�9mol��ʱת��24mol���� |
 ��
��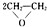 ��
��