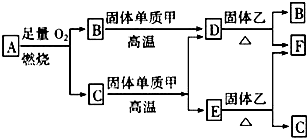
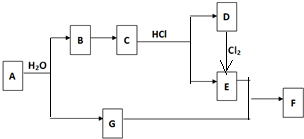
��Ŀ����
ʵ��������Ҫ����500mL 0.10mol?L-1��NaOH��Һ����ʵ��ش��������⣮
��1����������ƽ����NaOH��̬ g������NaOH������ע�������������⣺����ΪNaOH���и�ʴ�ԣ����Գ���ʱ��Ӧѡ�� ʢװNaOH���壻�ڳ�������Ѹ�٣�Ŀ���Ƿ�ֹ ��
��2��ʵ������Ҫ��������������ƽ��ҩ���⣬����Ҫ�IJ��������У� �� �� �� ��
��3�����в����������Ƶ���ҺŨ��û��Ӱ����� ��
A������ʱ���ӿ̶���
B�����ձ����ܽ�����Һ����ע������ƿ��Ȼ������������ˮ���̶���
C��ҡ�ȶ��ݺ��ý�ͷ�ι�������ƿ�еμ�����ˮ���̶���
D��������Һǰ������ˮ��ϴ����ƿ��
��1����������ƽ����NaOH��̬
��2��ʵ������Ҫ��������������ƽ��ҩ���⣬����Ҫ�IJ��������У�
��3�����в����������Ƶ���ҺŨ��û��Ӱ�����
A������ʱ���ӿ̶���
B�����ձ����ܽ�����Һ����ע������ƿ��Ȼ������������ˮ���̶���
C��ҡ�ȶ��ݺ��ý�ͷ�ι�������ƿ�еμ�����ˮ���̶���
D��������Һǰ������ˮ��ϴ����ƿ��
���㣺��Һ������
ר�⣺ʵ����
��������1������n=cv������������Ƶ����ʵ������ٸ���m=nM���������������Ƶ���������ʴ���׳����ҩƷӦ���ڲ���������Ѹ�ٳ�����
��2��������Һ�����������ϸ�����������ѡȡ������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
�����жϣ�
��2��������Һ�����������ϸ�����������ѡȡ������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
| n |
| v |
���
�⣺��1�����������Ƶ�����Ϊm=0.5L��0.1mol?L-1��40g/mol=2.0g��
���������и�ʴ���׳���Ӧ����С�ձ���Ѹ�ٳ�������ֹ���⣬
�ʴ�Ϊ��2.0��С�ձ������⣻
��2��������ƽ�������������������ƣ�����Ҫ��500ml����ƿ������0.10mol?L-1����Һ�����ձ����ܽ��̬�������ƣ��ò��������衢�������ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ��500ml����ƿ���ձ�����ͷ�ιܣ���������
��3��A������ʱ���ӿ̶��ߣ���ʹ���������ˮ���࣬������Һ���ƫ��������ҺŨ��ƫ�ͣ�
B����Һ�����������������ʣ����������ܽ���ȣ�δ��ȴ�����£����Ƚ���Һ��������ƿ�����������Һ���ᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ�
C��ҡ�Ⱥ�Һ���½���һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ�
D�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
�ʴ�Ϊ��D��
���������и�ʴ���׳���Ӧ����С�ձ���Ѹ�ٳ�������ֹ���⣬
�ʴ�Ϊ��2.0��С�ձ������⣻
��2��������ƽ�������������������ƣ�����Ҫ��500ml����ƿ������0.10mol?L-1����Һ�����ձ����ܽ��̬�������ƣ��ò��������衢�������ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ��500ml����ƿ���ձ�����ͷ�ιܣ���������
��3��A������ʱ���ӿ̶��ߣ���ʹ���������ˮ���࣬������Һ���ƫ��������ҺŨ��ƫ�ͣ�
B����Һ�����������������ʣ����������ܽ���ȣ�δ��ȴ�����£����Ƚ���Һ��������ƿ�����������Һ���ᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ�
C��ҡ�Ⱥ�Һ���½���һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ�
D�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
�ʴ�Ϊ��D��
���������⿼����Һ�����ƣ��ؼ�Ҫ������Һ���Ƶ�ԭ���Ͳ����Լ��������������ú�ʹ�÷�������Ҫ����ƿҪ������������������ͨ����ʽc=
�������⣮
| n |
| v |

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
�����Ŀ
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ�ǣ�������
| A����0.2 mol?L-1��NH4Al��SO4��2��Һ��0.3 mol?L-1��Ba��OH��2��Һ�������ϣ�2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� |
| B��NH4HCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3-+Ba2++OH-�TBaCO3��+H2O |
| C������������Һ��ϡ���ᡢ˫��ˮ��ϣ�Fe2++H2O2+2H+=Fe3++2H2O |
| D��������ʯͶ�뵽���������У�CO32-+2H+=CO2��+H2O |
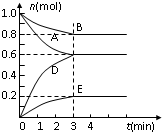 T�桢2L�ܱ�������ijһ��Ӧ��ͬʱ�̸����ʵ�����ͼ��EΪ���壬����Ϊ���壩���ش��������⣺
T�桢2L�ܱ�������ijһ��Ӧ��ͬʱ�̸����ʵ�����ͼ��EΪ���壬����Ϊ���壩���ش��������⣺