��Ŀ����
17����ҵ�Ϻϳ�a-��Ʒ��G��·��֮һ���£�
��֪��

R-OH+HBr��H2O��R-Br+H2O
��ش��������⣺
��1��F�����������ŵ�������������B��C�ķ�Ӧ����Ϊȡ����Ӧ��
��2��A�ĺ˴Ź���������4�����շ壬�����Ϊ1��1��4��4��
��3��д��C��Dת���Ļ�ѧ����ʽ
 ��
����4��д��B��һ�������¾ۺϳɸ߷��ӻ�����Ļ�ѧ����ʽ
 ��
����5��G������H2O���ӳɵõ�������H����д��H���ܵĽṹ��ʽ��дһ�ּ��ɣ�
 ��
����6��д��ͬʱ��������������E��ͬ���칹��Ľṹ��ʽ
 ��
����ֻ��3�ֻ�������ԭ�� ���ܷ���������Ӧ �۷����к���Ԫ����
���� ��A��B�Ľṹ��֪��A����鷢���ӳɷ�Ӧ����B��B��HBr����ȡ����Ӧ����CΪ ��C������ȥ��Ӧ����DΪ
��C������ȥ��Ӧ����DΪ ��D�ữ�õ�EΪ
��D�ữ�õ�EΪ ��E���Ҵ�����������Ӧ����FΪ
��E���Ҵ�����������Ӧ����FΪ ��F������Ϣ��Ӧ����G�����F��G�Ľ�Ͽ�֪YΪCH3MgBr���ݴ˽��
��F������Ϣ��Ӧ����G�����F��G�Ľ�Ͽ�֪YΪCH3MgBr���ݴ˽��
��� �⣺��A��B�Ľṹ��֪��A����鷢���ӳɷ�Ӧ����B��B��HBr����ȡ����Ӧ����CΪ ��C������ȥ��Ӧ����DΪ
��C������ȥ��Ӧ����DΪ ��D�ữ�õ�EΪ
��D�ữ�õ�EΪ ��E���Ҵ�����������Ӧ����FΪ
��E���Ҵ�����������Ӧ����FΪ ��F������Ϣ��Ӧ����G�����F��G�Ľ�Ͽ�֪YΪCH3MgBr��
��F������Ϣ��Ӧ����G�����F��G�Ľ�Ͽ�֪YΪCH3MgBr��
��1��FΪ ��F�����������ŵ�������������B��C�ķ�Ӧ����Ϊ ȡ����Ӧ��
��F�����������ŵ�������������B��C�ķ�Ӧ����Ϊ ȡ����Ӧ��
�ʴ�Ϊ�������� ȡ����Ӧ��
��2������A�Ľṹ��ʽ��֪��A�ĺ˴Ź���������4�����շ壬�����Ϊ 1��1��4��4��
�ʴ�Ϊ��4��1��1��4��4��
��3��C��D�Ļ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
��4��B�к����ǻ����Ȼ�����B��һ�������¾ۺϳɸ߷��ӻ�����Ļ�ѧ����ʽ ��
��
�ʴ�Ϊ�� ��
��
��5������G�Ľṹ��ʽ��֪��G����̼̼˫����G������H2O���ӳɵõ�������HΪ  ��
��
�ʴ�Ϊ�� ��
��
��6��EΪ ������������ֻ��3�ֻ�������ԭ�ӣ�˵�����ӽṹ��Ϊ�Գƣ����ܷ���������Ӧ���ܷ���������Ӧ��˵����ȩ�����۷����к���Ԫ���������������E��ͬ���칹��Ľṹ��ʽΪ
������������ֻ��3�ֻ�������ԭ�ӣ�˵�����ӽṹ��Ϊ�Գƣ����ܷ���������Ӧ���ܷ���������Ӧ��˵����ȩ�����۷����к���Ԫ���������������E��ͬ���칹��Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶ���ϳɣ��Ѷ��еȣ���������л���Ľṹ����Ӧ��������Ӧ��Ϣ�����жϣ����չ����ŵ����������ⷴӦ��Ϣ�ǹؼ����ܽϺõĿ��鿼�����Ķ�����ѧ������˼ά���������ȵ����ͣ�

| A�� | R�ĵ�����ͨ������³���̬ | B�� | R�ĵ��ʿ�����һ����ɫ�Ĺ��� | ||
| C�� | R���⻯���ȶ� | D�� | ����R��H2�Ļ��Ϸ�Ӧ�dz����� |
| A�� | NA������������NA���������ӵ���������4��5 | |
| B�� | 17gOH-��19gH3O+������������� | |
| C�� | 32gSO2��40gSO3������ԭ������� | |
| D�� | ��״���£�11.2LCO2�볣�³�ѹ��17gNH3������������� |



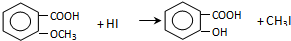 ��
��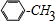 +Cl2$\stackrel{����}{��}$
+Cl2$\stackrel{����}{��}$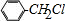 +HCl����Ӧ����Ϊȡ����Ӧ��
+HCl����Ӧ����Ϊȡ����Ӧ��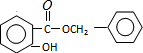 ��
�� ����
����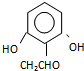 ������дһ�ּ��ɣ�
������дһ�ּ��ɣ� 

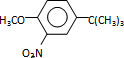 ����Ӧ����3���õ��Լ�ΪŨHI��
����Ӧ����3���õ��Լ�ΪŨHI�� ��MX��ˮ��Ӧ�ɷų����壬�÷�Ӧ�Ļ�ѧ����ʽΪNaH+H2O=NaOH+H2����
��MX��ˮ��Ӧ�ɷų����壬�÷�Ӧ�Ļ�ѧ����ʽΪNaH+H2O=NaOH+H2����
 ��R�����
��RΪ����� ��E�к��еĹ������������ǻ���ȩ����
��E�к��еĹ������������ǻ���ȩ���� $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$ +H2O���÷�Ӧ����Ϊ��ȥ��Ӧ��
+H2O���÷�Ӧ����Ϊ��ȥ��Ӧ�� +CH3CH2CH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$
+CH3CH2CH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$ +H2O���÷�Ӧ����Ϊ������Ӧ��
+H2O���÷�Ӧ����Ϊ������Ӧ�� ��ѧ�о�������ͭ�������CuMn2O4�����ڳ����´����������е�һ����̼�ͼ�ȩ��HCHO��
��ѧ�о�������ͭ�������CuMn2O4�����ڳ����´����������е�һ����̼�ͼ�ȩ��HCHO�� ��
�� N2H4���£���������ҩ��ԭ�ϣ�Ҳ���������ȼ�ϣ���1���������ᷴӦ��N2H6Cl2��Һ�������ԣ���ˮ�д������·�Ӧ��
N2H4���£���������ҩ��ԭ�ϣ�Ҳ���������ȼ�ϣ���1���������ᷴӦ��N2H6Cl2��Һ�������ԣ���ˮ�д������·�Ӧ�� ��
�� ��
�� ��
�� ��
�� ��
��