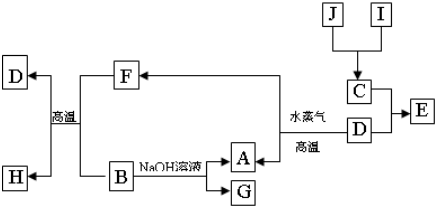
��Ŀ����
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������ֻ��CΪ����Ԫ�أ�A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ����������͵�2����������Ԫ�صĵ�������2�����壬2�ֹ��塣
��1��д��Ԫ�ط��ţ�A ��B ��C ��D ��
��2������A��B��C��D����Ԫ��������ɵĶ�Ԫ�������У��Ǽ��Է����� ����ṹ�д��ڷǼ��Լ��ķ��Ӿ����� ����ṹ�д��ڷǼ��Լ������Ӿ�����__________��������һ�����ʵĻ�ѧʽ��
��3��д�����־�����A��B��C��D����Ԫ�صĻ��������֣� �� ��
��1�� H O Na S ��2��SO3 H2O2 Na2O2��3�� NaHSO3 NaHSO4
���������������1��A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ����������͵�2��������������Ԫ���з�����������ֻ��H��Na��O��S����A��B��C��D�ֱ�ΪH��O��Na��S��
��2������A��B��C��D����Ԫ��������ɵĶ�Ԫ�������У��Ǽ��Է���������������ṹ�д��ڷǼ��Լ��ķ��Ӿ�����˫��ˮ����ṹ�д��ڷǼ��Լ������Ӿ����й������ơ�
��3������H��O��Na��S����Ԫ�صĻ�����ֱ���NaHSO4�� NaHSO3��
���㣺����Ԫ�����ڱ��Ľṹ����ѧ�������Ӽ��ԡ���ѧʽ���й��ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬���������ǿ�����ض�ѧ�����������������֪ʶ�Ĺ�����ѵ����������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ�������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ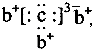 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
 Al��OH��3+OH-
Al��OH��3+OH-