��Ŀ����
ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ⶨδ֪Ũ�ȵ����ᣬ�����Dzⶨ��ʵ��������森������д�йؿհף�
��1���ⶨĿ�ģ��ñ�NaOH��Һ�ⶨ�����Ũ��
��2���ⶨԭ����NaOH+HCl=NaCl+H2O
��3��ʵ����Ʒ���Լ�
����������Ʒ����ʽ�ζ��ܡ���ʽ�ζ��ܡ���ƿ���ζ��ܵ�
���Լ���Ũ��0.2000mol?L-1�ı�NaOH��Һ����̪��Һ������ˮ�ȣ�
��4��ʵ�����
�ټ��ζ����Ƿ�©Һ��ϴ����������ϴ��װ�ñ�NaOH��Һ���ã�
���� ���������ƣ�����ƿ������20.00mL����Ũ�ȵ����ᣬ����2��ָʾ����
������������װ��NaOH��Һ�ζ���Һ�����̶ȡ�0����0���̶����£�
�������ֿ��Ƶζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��ֱ���ζ��յ㣮�ζ����յ�ʱ�������� ��
��5�����ݼ�¼�봦�������������������������NaOH��Һ��ƽ�������������������Ũ�ȣ�������Ӧ�Ļ����ϣ���
ͨ������ɵã�������Ũ��Ϊ�� mol?L-1������������2λС����
��6���������ۣ�
������ʵ����̣�1������������ˮϴ�Ӻ�δ�ñ�NaOH��Һ��ϴ���ᵼ�²ⶨ������ƫ����ƫС������Ӱ�족��
�ڲ��衰ʵ����̣�2�����У�����ƿװҺǰ��������������ˮ���ⶨ������ƫ����ƫС������Ӱ�족�� ��
��1���ⶨĿ�ģ��ñ�NaOH��Һ�ⶨ�����Ũ��
��2���ⶨԭ����NaOH+HCl=NaCl+H2O
��3��ʵ����Ʒ���Լ�
����������Ʒ����ʽ�ζ��ܡ���ʽ�ζ��ܡ���ƿ���ζ��ܵ�
���Լ���Ũ��0.2000mol?L-1�ı�NaOH��Һ����̪��Һ������ˮ�ȣ�
��4��ʵ�����
�ټ��ζ����Ƿ�©Һ��ϴ����������ϴ��װ�ñ�NaOH��Һ���ã�
����
������������װ��NaOH��Һ�ζ���Һ�����̶ȡ�0����0���̶����£�
�������ֿ��Ƶζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��ֱ���ζ��յ㣮�ζ����յ�ʱ��������
��5�����ݼ�¼�봦�������������������������NaOH��Һ��ƽ�������������������Ũ�ȣ�������Ӧ�Ļ����ϣ���
| �ζ����� | �������mL | NaOH��Һ���������mL�� | ����NaOH��Һ��ƽ�������mL�� | |
| �ζ�ǰ | �ζ��� | |||
| 1 | 20.00 | 0.00 | 16.26 | |
| 2 | 20.00 | 0.00 | 16.30 | |
| 3 | 20.00 | 0.02 | 16.24 | |
��6���������ۣ�
������ʵ����̣�1������������ˮϴ�Ӻ�δ�ñ�NaOH��Һ��ϴ���ᵼ�²ⶨ������ƫ����ƫС������Ӱ�족��
�ڲ��衰ʵ����̣�2�����У�����ƿװҺǰ��������������ˮ���ⶨ������ƫ����ƫС������Ӱ�족��
���㣺�к͵ζ�
ר�⣺ʵ����
��������4����ȷ��ȡ20.00mL�������ᣬ������ʽ�ζ��ܻ���Һ�ܣ�
�ܸ�����Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��
��5�����ж����ݵĺ����ԣ�Ȼ��������Һ��ƽ�������Ȼ�����HCl��NaOH��HCl��Һ�����ʵ���Ũ�ȣ�
��6������c�����⣩=
��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
�ܸ�����Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��
��5�����ж����ݵĺ����ԣ�Ȼ��������Һ��ƽ�������Ȼ�����HCl��NaOH��HCl��Һ�����ʵ���Ũ�ȣ�
��6������c�����⣩=
| V(��)��c(��) |
| V(����) |
���
�⣺��4��������ʽ�ζ��ܻ���Һ�ܣ����������ƣ�����ƿ������20.00mL����Ũ�ȵ����ᣬ�ʴ�Ϊ����ʽ�ζ��ܻ���Һ�ܣ�
�ܵζ��յ�ı�־Ϊ�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��
�ʴ�Ϊ�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��
��5���������ĵı�NaOH��Һ�����Ϊ16.26mL��16.30mL��16.22mL������Ч����NaOH��Һ��ƽ�����Ϊ16.28mL��
HCl��NaOH
1 1
c��HCl����20.00 0.2000mol?L-1��16.28mL
��ã�c��HCl��=0.16mol/L��
�ʴ�Ϊ��0.16��
��6��������ʵ����̣�1������������ˮϴ�Ӻ�δ�ñ�NaOH��Һ��ϴ����Һ�൱��ϡ�ͣ����V������ƫ����c�����⣩=
��������֪c�����⣩ƫ�ʴ�Ϊ��ƫ��
�ڲ��衰ʵ����̣�2�����У�����ƿװҺǰ��������������ˮ������Һ�����ʵ������䣬V���������䣬����c�����⣩=
��������֪c�����⣩��Ӱ�죬�ʴ�Ϊ����Ӱ�죮
�ܵζ��յ�ı�־Ϊ�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��
�ʴ�Ϊ�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ��
��5���������ĵı�NaOH��Һ�����Ϊ16.26mL��16.30mL��16.22mL������Ч����NaOH��Һ��ƽ�����Ϊ16.28mL��
HCl��NaOH
1 1
c��HCl����20.00 0.2000mol?L-1��16.28mL
��ã�c��HCl��=0.16mol/L��
�ʴ�Ϊ��0.16��
��6��������ʵ����̣�1������������ˮϴ�Ӻ�δ�ñ�NaOH��Һ��ϴ����Һ�൱��ϡ�ͣ����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
�ڲ��衰ʵ����̣�2�����У�����ƿװҺǰ��������������ˮ������Һ�����ʵ������䣬V���������䣬����c�����⣩=
| V(��)��c(��) |
| V(����) |
������������Ҫ�������к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ���ǽ���ؼ���

��ϰ��ϵ�д�
 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
�����й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
A�� ���ô�ͼװ�ÿɴ��Ȼ�����Һ��ֱ�������ᾧ����Ȼ������� |
B�� ���ô�ͼװ�ÿɷ���ʯ�ͣ��õ����͡�ú�ͺͲ��͵ȸ������ |
C��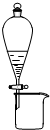 ���ô�ͼװ�ÿɷ���CH3CH2OH��CH3COOC2H5���Һ |
D�� ���ô�ͼװ�ÿɽ�������к͵ζ� |
��NAΪ�����ӵ�������ֵ������������ȷ���ǣ�������
| A����״���£�11.2L�Ҵ��������ǻ���Ϊ0.5 NA |
| B�����³�ѹ�£�4.6g NO2��N2O4������к��е�ԭ������Ϊ0.3 NA |
| C��1mol Cl2������Ӧʱ��ת�Ƶĵ�����һ����2NA |
| D�������£�1L pH=12��Ba��OH��2��Һ�к��е�OH-������Ϊ0.02NA |
 ���ྻ�����ֽ���ƬX��Y��Z�ֱ�����ڽ���ʳ����Һ����ֽ�ϲ�ѹ������ͼ����ÿ��ʵ��ʱ����ѹ��ָ���ƫ�Ʒ���Ͷ������±�����֪�������缫�Ľ��������������Խ�����ѹԽ��X��Y��Z��ͭ���ֽ���������˵��������ȷ���ǣ�������
���ྻ�����ֽ���ƬX��Y��Z�ֱ�����ڽ���ʳ����Һ����ֽ�ϲ�ѹ������ͼ����ÿ��ʵ��ʱ����ѹ��ָ���ƫ�Ʒ���Ͷ������±�����֪�������缫�Ľ��������������Խ�����ѹԽ��X��Y��Z��ͭ���ֽ���������˵��������ȷ���ǣ�������| ����Ƭ | �������� | ��ѹ��V�� |
| X | X��Cu | +0.78 |
| Y | Cu��Y | -0.15 |
| Z | Z��Cu | +1.35 |
| A��Z����������������������������������Y���� |
| B��Y�������ܴ�������Һ���û������� |
| C�����ֽ����Ļ�����˳��Ϊ��Z��X��Y |
| D��X��Y�ܹ��ɵ�ѹ����ԭ��� |
NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A��7.8g Na2O2�к��е���������ĿΪ0.4NA |
| B��1mol�������к���̼̼˫����ĿΪ3NA |
| C����״���£�����������ΪNA��NH3��HCl�����Ϻ�����ԼΪ22.4L |
| D��16g CH4��18g NH4+������������Ϊ10NA |