��Ŀ����
17��ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�������Ũ����ʵ�����н�������ʵ�飺I������100mL 0.10mol/L NaOH����Һ��
II��ȡ20.00mL����ϡ���������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�
NaOH��Һ���еζ���
III���ظ������ζ�����2��3�Σ���¼�������£�
| ʵ�� ��� | NaOH��Һ��Ũ�ȣ�mol/L�� | �ζ����ʱ��NaOH��Һ����������mL�� | ����HCl��Һ�������mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
��2�������������ݣ��ɼ�����������Ũ��ԼΪ0.11mol/L��������λ��Ч���֣���
��3����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ���IJ�����Ȼ��ѹ������ʹ���첿�ֳ�����Һ��

��4��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���EF����ѡ�۷֣���
A���ζ��յ����ʱ���Ӷ��� B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C����ƿˮϴ��δ���� D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��
���� ��1������ָʾ��Ϊ��̪���ζ�����ǰ��ҺΪ��ɫ���ζ�����ʱ��Һ��ɺ�ɫ�����жϵζ��յ㣻
��2�����ݵζ����ĵı�Һ������ж����ݵ���Ч�ԣ�Ȼ��������Һ��ƽ������������ݱ�c�����⣩=$\frac{c����V��}{V����}$������������Ũ�ȣ�
��3��������ȷ��ȥ��ʽ�ζ��������ݵķ������з�����
��4�����ݲ�����c�����⣩=$\frac{c����V��}{V����}$��Ӱ������ܹ����²ⶨ���ƫ�ߵ�ѡ�
��� �⣺��1���ζ�����ǰ�����е����̪����ҺΪ��ɫ���ζ�����ʱ�������ƹ�������Һ��ɺ�ɫ�����Եζ��յ�����Ϊ�����һ������������Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��
�ʴ�Ϊ�����һ������������Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ɫ��ɺ�ɫҲ�ɣ���
��2�����εζ����ݶ�����Ч�ģ����ı�Һ��ƽ�����Ϊ��$\frac{22.62+22.72+22.80}{3}$mL=22.713mL��
�������Ũ��Ϊ��c��HCl��=$\frac{c����V��}{V����}$=$\frac{0.1mol/L��0.022713L}{0.02L}$��0.11mol/L��
�ʴ�Ϊ��0.11mol/L��
��3����ʽ�ζ���װ����Һ����Ĵָ��ʳָ��ס���������ڲ�λ��ʹ�齺���������������ڹ�б���ϣ�Ȼ���ڲ�����λ����Ѹ������Ƥ�ܣ�ʹ��Һ�ӹܿ���������Ա���ȷ��
�ʴ�Ϊ������
��4��A���ζ��յ����ʱ���Ӷ������������ı�Һ���������ƫ�ͣ�����c�����⣩=$\frac{c����V��}{V����}$��֪���ⶨ���ƫ�ͣ���A����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ�����´���ҺŨ�ȼ�С�����ĵı�Һ�����С������c�����⣩=$\frac{c����V��}{V����}$�ƿ�֪���ⶨ���ƫ�ͣ���B����
C����ƿˮϴ��δ����Ա�Һ�����ʵ�����Ӱ�죬�ζ�ʱ�Ա�Һ�����û��Ӱ�죬����c�����⣩=$\frac{c����V��}{V����}$�ƿ�֪���ⶨ������䣬��C����
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ�У����V������ƫС������c�����⣩=$\frac{c����V��}{V����}$��֪������ҺŨ��ƫ�ͣ���D����
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶��ߣ�����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c����V��}{V����}$��֪������ҺŨ��ƫ��E��ȷ��
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���������ĵı�Һ�������ƫ����c�����⣩=$\frac{c����V��}{V����}$�ƿ�֪���ⶨ���ƫ�ߣ���F��ȷ��
��ѡEF��
���� ���⿼�����к͵ζ�����������Ҫ��ѡ�������к͵ζ��IJ�����������ɷ����ķ��������������ǿ�������߿��������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�ijʵ��С����������к͵ζ����ⶨʳ����������CH3COOH��g/100mL����
��һ����ʵ����Ʒ������ʳ�ð״���Ʒ500mL���̱�ע������������3.5g-5g/100mL�����³ơ�ԭ�ס�����0.1000mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��������ѡ��
��������ʵ��ԭ������ѧ����ʽCH3COOH+NaOH=CH3COONa+H2O
��������ʵ�鲽�裺
��1�����ƴ���ʳ����Һ
ȷ��ȡ10.00mLԭ�ף����Ƴ�100.00mL����ʳ����Һ���³ơ�����Һ��������ɸò��裬��Ҫ��һ�ֶ���������100ml����ƿ�����������ƣ�
��2����ȡһ������Ĵ���Һ
����ʽ�ζ���ȡ20.00mL����Һ����ƿ�У���1-2��0.1%��̪��Һ��ָʾ��
��3��ʢװ��NaOH��Һ����¼ΪNaOH����Һ����ij�������
��4���ζ��������ζ�ʱһ�ֿ��Ƶζ��ܣ�һ��ҡ����ƿ���۾��۲���ƿ����Һ��ɫ�仯ֱ���ζ��յ㣮�жϵζ��յ������������ɫ��Ϊdz��ɫ��������ڲ���ɫ��
��¼NaOH���ն������ظ��ζ�4�Σ�
���ģ���ʵ���¼�����ݴ���������������գ�
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V������Һ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.95 | 15.20 | 15.15 | 16.95 |
| V��NaOH��/mL�����ģ� | 14.95 | 15.00 | 15.05 | 16.95 |
��6������NaOH��Һ��ƽ��ֵ��15.00mL��
��7������Һ��Ũ����0.075mol/L��
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
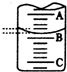
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ25.40mL����ʱ�ζ�����Һ����� ������24.60ml��
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
A��ʵ�����ʱ���ӵζ�����Һ�棬��ȡ�ζ��յ�ʱNaOH��Һ�����
B���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
C��ʢװ��Һ�ĵζ���װҺǰ������ˮϴ�ӣ�δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
��4�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ�����ػ���c��CH3COOH��=$\frac{c����25.35+25.30��}{2V}$mol/L��
| A�� | �����巢��װ���ϵ�ȼ����������ʱ�������ȼ�������Ĵ��� | |
| B�� | �������պ�����������ʵ��������ȴ������ | |
| C�� | ����С�Ĵƾ���ʹ�ƾ��Ż�ʱ��Ӧ��ʪĨ������ | |
| D�� | ������������У����������ӷ�ʯ��Ӧ����ֹͣ���ȣ�����ƿ��ȴ���ټ����ʯ |
 ��һ���ѳ�ȥ��������Ĥ�����������������Թ���ڣ���ͼ�������Թܽ������ṯ��Һ�У�Ƭ��ȡ����Ȼ�����ڿ����У���������������������ë������īˮ���Ҷ�����������ʵ�������ж�����˵��������ǣ�������
��һ���ѳ�ȥ��������Ĥ�����������������Թ���ڣ���ͼ�������Թܽ������ṯ��Һ�У�Ƭ��ȡ����Ȼ�����ڿ����У���������������������ë������īˮ���Ҷ�����������ʵ�������ж�����˵��������ǣ�������| A�� | ʵ���з����ķ�Ӧ���ǻ��Ϸ�Ӧ | B�� | ����һ�ֽϻ��õĽ��� | ||
| C�� | ����������Ӧ�ų����������� | D�� | ��Ƭ�����ɵİ�ë�������� |
 ���嶡�����ӣ�
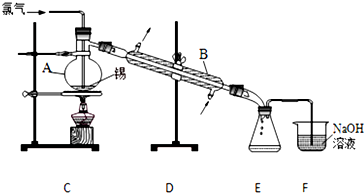
���嶡�����ӣ�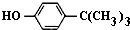 ����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ�
����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ� ��
��
 ij��ѧѧϰС����о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O����x��ֵ������ͬѧͨ���������ϲ�Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
ij��ѧѧϰС����о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O����x��ֵ������ͬѧͨ���������ϲ�Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���