��Ŀ����
8�� ij��ѧ����ʵ��С�������ͼʾʵ��װ������ȡ�屽��֤���÷�ӦΪȡ����Ӧ����֪���屽���۵�Ϊ?30.8�棬�е�156�森
ij��ѧ����ʵ��С�������ͼʾʵ��װ������ȡ�屽��֤���÷�ӦΪȡ����Ӧ����֪���屽���۵�Ϊ?30.8�棬�е�156�森��1��������ƿ�з������л���Ӧ��ѧ����ʽ
 ��
����2������A�����ƣ�ֱ�Σ������ܣ�
��ʵ��ʹ������A��ԭ��������������ȴ��Ӧ��ϵ���ʣ���ֹ��Ӧ���Ȼӷ����죮����ʵ����ʵ��ʵ��ʱ��û��ʹ��A��ԭ�����²����ը�Ѳ���������
��3����ƿ��С�Թ���CCl4�������ջӷ����壻�粻�Ӹò���װ�ý�����ʲô�����˵��ȡ����Ӧ����
��4����Һ©���ڵ�NaOH��Һ���ã���ȥ�ܽ����屽�е��壮
���� ��ȡ�屽��֤���÷�ӦΪȡ����Ӧ����ʵ��װ�ÿ�֪��������ƿ��Fe���巴Ӧ�����廯���������巢��ȡ����Ӧ�����屽��HBr������AΪ�����ܣ�������������Ӧ�ʹ��Ӧ���ַ�Ӧ��NaOH��Һ�ɳ�ȥʣ����嵥�ʣ���ƿ��С�Թ���CCl4�ɷ�ֹ�����������ջӷ����壬�Դ������
��1������Һ����Fe�Ĵ������·���ȡ����Ӧ�����屽���廯�⣻
��2����������A�Ľṹ�ص��Լ��������������ƽ���ڷ�Ӧ�������ܵ��������������������������ã�ʵ��ʵ��ʱ��û��ʹ��A����ֹ�²����ը�Ѳ���������
��3���������ӷ�����ƿ��С�Թ���CCl4���������ջӷ������������粻�Ӹò���װ�ã�Br2��H2O��ӦҲ������HBr����˵��ȡ����Ӧ������
��4������Һ�ܺ��巴Ӧ�����屽����Ӧ���������屽�����ܣ�
��� �⣺��1��������ƿ��Fe���巴Ӧ�����廯���������巢��ȡ����Ӧ�����屽��HBr���л���ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2������AΪ�����ܣ��������ܵ�����Ϊֱ�Σ�����A������Ϊ��ֱ�Σ������ܣ�����Ϊ������������ȴ��Ӧ��ϵ���ʣ���ֹ��÷�Ӧ���Ȼӷ����죬����ʵ����ʵ��ʵ��ʱ��û��ʹ��A��ԭ���Ƿ�ֹ���²����ը�Ѳ���������
�ʴ�Ϊ����ֱ�Σ������ܣ�������������ȴ��Ӧ��ϵ���ʣ���ֹ��Ӧ���Ȼӷ����죻�²����ը�Ѳ���������
��3����ƿ��С�Թ���CCl4�����ǣ����ջӷ����壬�粻�Ӹò���װ�ã�Br2�ӷ���Br2��H2O��ӦҲ������HBr����˵��ȡ����Ӧ������
�ʴ�Ϊ�����ջӷ����壻��˵��ȡ����Ӧ������
��4����ȥ�屽�л��е�Br2���ʵ��Լ����������ƣ�2NaOH+Br2�TNaBr+NaBrO+H2O��NaBr��NaBrO���屽�����ܣ�������������ƿ�м�����������������Һ����ת���Һ©������Һ���ɣ�
�ʴ�Ϊ����ȥ�ܽ����屽�е��壮
���� ���⿼���屽���Ʊ�ʵ�飬Ϊ��Ƶ���㣬���շ����ķ�Ӧ��ʵ��װ�õ����á�ʵ�鼼�ܵ�Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע���л�������ʼ����������ᴿ��Ӧ�ã���Ŀ�Ѷ��еȣ�

 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�| A�� | AgCl��AgI��AgBr | B�� | AgCl��AgBr��AgI | C�� | AgBr��AgCl��AgI | D�� | AgBr��AgI��AgCl |
| A�� | c1=2c2 | B�� | c1��c2 | C�� | c1=c2 | D�� | c1��c2 |
| A�� | 0.2 mol | B�� | 0.14mol | C�� | 4g | D�� | 5.6g |



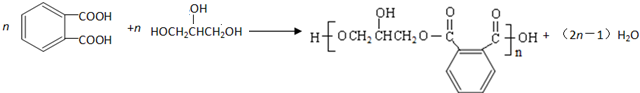
 �ķ�������������ͬ���칹����6 �֣������������칹��
�ķ�������������ͬ���칹����6 �֣������������칹��