��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش���������
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ ��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳���� ��
��3��������ӽṹʾ��ͼ ��
��4����ͬԪ�ص�ԭ���ڷ������������ӵ�������С���õ縺��x����ʾ����xԽ��˵����Ԫ�طǽ�����Խǿ���Ƚ�x���ࣩ x���ᣩ���������=����д��һ��֧����һ�Ƚϵ�ʵ����ʵ ��
��5��д��ͭ��۵�����������Ӧˮ�����ϡ��Һ��Ӧ�����ӷ��� ��
| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����
��3��������ӽṹʾ��ͼ
��4����ͬԪ�ص�ԭ���ڷ������������ӵ�������С���õ縺��x����ʾ����xԽ��˵����Ԫ�طǽ�����Խǿ���Ƚ�x���ࣩ
��5��д��ͭ��۵�����������Ӧˮ�����ϡ��Һ��Ӧ�����ӷ���
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
����������Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢޢߢ��ֱ���H��C��N��O��Na��Al��Si��S��ClԪ�أ�
��1��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��
��2��Ԫ�طǽ�����Խǿ��������������ˮ��������Խǿ��
��3�������Ӻ�����3�����Ӳ㡢�����8�����ӣ�������16�����ӣ�
��4��ͬһ���ڣ�Ԫ�طǽ���������ԭ�������������ǿ���ݴ��ж���縺�Դ�С�������û���Ӧ�жϷǽ�����ǿ����
��5���۵�����������ˮ������HNO3��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ��
��1��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��
��2��Ԫ�طǽ�����Խǿ��������������ˮ��������Խǿ��
��3�������Ӻ�����3�����Ӳ㡢�����8�����ӣ�������16�����ӣ�
��4��ͬһ���ڣ�Ԫ�طǽ���������ԭ�������������ǿ���ݴ��ж���縺�Դ�С�������û���Ӧ�жϷǽ�����ǿ����
��5���۵�����������ˮ������HNO3��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ��
���
�⣺����Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢޢߢ��ֱ���H��C��N��O��Na��Al��Si��S��ClԪ�أ�
��1��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С������������Ԫ��ԭ�Ӱ뾶��С˳����Na��Al��O���ʴ�Ϊ��Na��Al��O��
��2���ǽ�����N��C��Si�����������ַǽ����������ˮ��������ǿ��˳����HNO3��H2CO3��H2SiO3����H4SiO4�����ʴ�Ϊ��HNO3��H2CO3��H2SiO3����H4SiO4����
��3�������Ӻ�����3�����Ӳ㡢�����8�����ӣ�������16�����ӣ����������ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4���ǽ�����S��Cl����縺��S��Cl���ǽ����ķǽ�����Խǿ���䵥�ʵ�������Խǿ������Cl2+H2S=S+2HCl֪�������������Դ���S����Cl�ĵ縺�Դ���S���ʴ�Ϊ���������������ⷴӦ���������������𰸾��ɣ���
��5���۵�����������ˮ������HNO3��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��1��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С������������Ԫ��ԭ�Ӱ뾶��С˳����Na��Al��O���ʴ�Ϊ��Na��Al��O��
��2���ǽ�����N��C��Si�����������ַǽ����������ˮ��������ǿ��˳����HNO3��H2CO3��H2SiO3����H4SiO4�����ʴ�Ϊ��HNO3��H2CO3��H2SiO3����H4SiO4����
��3�������Ӻ�����3�����Ӳ㡢�����8�����ӣ�������16�����ӣ����������ӽṹʾ��ͼΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4���ǽ�����S��Cl����縺��S��Cl���ǽ����ķǽ�����Խǿ���䵥�ʵ�������Խǿ������Cl2+H2S=S+2HCl֪�������������Դ���S����Cl�ĵ縺�Դ���S���ʴ�Ϊ���������������ⷴӦ���������������𰸾��ɣ���
��5���۵�����������ˮ������HNO3��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
���������⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã��漰���ʵ����ʡ�Ԫ�������ɡ���ѧ�����֪ʶ�㣬��������Ԫ�ػ�����֪ʶ���������Ԫ�������ɼ��ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
����������ȷ���ǣ�������
| A�������£�0.01mol/LCH3COOH��pH=12��NaOH��Һ��ϣ�����Ϻ�c��CH3COO-����c��Na+��������Һһ���ʼ��� |
| B�������£��������0.01mol/LHCl��pH=12�İ�ˮ��ϣ�����Һ��pH=7 |
| C��0.1mol/L��ij��Ԫ������Na2A��Һ�У�c��Na+��=2c��H2A��+2c��HA?��+2c��A2-�� |
| D����5 mL 0.02mol/L��H2SO4��5 mL 0.02mol/LNaOH��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ10mL������Һ��pH=2 |
��NA��ʾ�����ӵ�����������˵��������ǣ�������
| A��һ������Fe�뺬1 mol HNO3��ϡ����ǡ�÷�Ӧ����ԭ�ĵ�ԭ����С��NA |
| B��1 L 1 mol/L��FeCl3��Һ�к���NA��Fe3+ |
| C�����³�ѹ�£���������SO2��S2������ͬ�ķ����� |
| D��125 g CuSO4?5H2O�������0.5NA��Cu2+ |
 ��Ҫ��ش���������
��Ҫ��ش���������


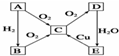 ��֪��AΪ�����к������ĵ��ʣ�������ͼת����ϵ���ش��������⣺
��֪��AΪ�����к������ĵ��ʣ�������ͼת����ϵ���ش��������⣺