��Ŀ����
16������ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ�����£�
�����õ����й��������£�
| ��Է������� | �ܶȣ�g/cm3�� | �е�/�� | �ܽ��� | |
| ������ | 100 | 0.9618 | 161 | ����ˮ |
| ����ϩ | 82 | 0.8102 | 83 | ������ˮ |
��a�м���20g��������2СƬ���Ƭ����ȴ��������������1mLŨ���ᣮb��ͨ����ȴˮ��ʼ��������a�������������¶Ȳ�����90�森
�����ᴿ��
��Ӧ�ֲ��ﵹ���Һ©���зֱ�������5%̼������Һ��ˮϴ�ӣ�����������ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�����ͨ������õ���������ϩ10g��
�ش��������⣺
��1��װ��a��������������ƿ��
��2���������Ƭ�������Ƿ�ֹ���У��������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������B������ȷ�𰸱�ţ���
A���������� B����ȴ�� C�����貹�� D����������
��3����Һ©����ʹ��ǰ����ϴ�ɾ�����©���ڱ�ʵ���������У�����Ӧ�ôӷ�Һ©�����Ͽڵ�������Ͽڵ��������¿ڷų�������
��4�������ᴿ�����м�����ˮ�Ȼ��Ƶ�Ŀ��������ˮ��
��5���ڻ���ϩ�ֲ�����������У������õ���������ABE������ȷ�𰸱�ţ���
AԲ����ƿ B�¶ȼ� C©�� D������ E������
��6����ʵ�����õ��Ļ���ϩ������61%����������λ��Ч���֣�
��ע������=$\frac{ʵ�ʲ���}{���۲���}$��100%��
���� ��1�����������ṹ�����ж�����a�����ƣ�
��2�����Ƭ�Ĵ��ڿ��Է�ֹ�ڼ��ȹ����в����������������Ƭʱ��Ҫ���Ѽ��ȵ���Һ��ȴ���ټ��룻
��3�����ڷ�Һ©���л������أ���ʹ��ǰ��Ҫ����Ƿ�©Һ����Һ�����У��ܶȴ��Һ̬�ӷ�Һ©�����¿ڷų����ܶ�С�Ĵ��Ͽڵ�����
��4����ˮ�Ȼ����������ղ�����������ˮ��
��5�������������������ϩ����ҪԲ����ƿ���¶ȼơ��������ȣ��ݴ��ж�ʹ�õ�������
��6��������Ϊ0.2mol�������Ͽ��Եõ�0.2mol����ϩ��������Ϊ16.4g�����ݲ���=$\frac{ʵ�ʲ���}{���۲���}$��100%���㣮
��� �⣺��1����������a�Ĺ����֪������Ϊ������ƿ��
�ʴ�Ϊ��������ƿ��
��2�����Һ̬�ڼ���ʱ���������������Ƭ�Ĵ��ڿ��Է�ֹ�ڼ��ȹ����в������������������һ��ʱ��������ǼӴ�Ƭ���������Ƭʱ��Ҫ���Ѽ��ȵ���Һ��ȴ���ټ��룬��B��ȷ��
�ʴ�Ϊ����ֹ���У�B��
��3�����ڷ�Һ©���л������أ���ʹ��ǰ��Ҫ����Ƿ�©Һ����Һ�����У����ڻ���ϩ���ܶȱ�ˮ���ܶ�С����Ӧ�ôӷ�Һ©�����Ͽڵ�����
�ʴ�Ϊ����©���Ͽڵ�����
��4����ˮ�Ȼ��ƾ�����ˮ�ԣ������ᴿ�����м�����ˮ�Ȼ��ƿ����ղ�����������ˮ��
�ʴ�Ϊ������ˮ��
��5�������������������ϩ����Ҫ�õ��������У�Բ����ƿ���¶ȼơ��������������ܵȣ������õ���������©����������ȷ��ΪABE��
�ʴ�Ϊ��ABE��
��6��20g�����������ʵ���Ϊ��$\frac{20g}{100g/mol}$=0.2mol�����ݷ�Ӧ����ʽ��֪�����Ͽ��Եõ�0.2mol����ϩ��0.2mol����ϩ������Ϊ��0.2mol��82g/mol=16.4g��
���Ի���ϩ�IJ���Ϊ��$\frac{10g}{16.4g}$��100%=61%��
�ʴ�Ϊ��61%��
���� ���⿼���л����Ʊ�ʵ�鷽������ƣ��漰��ѧ����ʶ�𡢻������������ʵķ����ᴿ�����ʼ����֪ʶ����Ŀ�Ѷ��еȣ���ȷʵ��ԭ����ʵ������ǽⱾ��ؼ�������������ѧ���ķ�����������������ѧʵ�顢��ѧ����������

| A�� | ������蝹����Ͻ����ڻ���� | |
| B�� | ${\;}_{75}^{185}$Re��${\;}_{75}^{187}$Re�������� | |
| C�� | 画�Re��Ԫ�ص����ԭ������Ϊ186 | |
| D�� | ��諸Ͻ�ȴ�蝹��۵�ߡ�Ӳ�ȴ� |
| A�� | 1 L 1 mol•L-1��NaClO ��Һ�к���ClO-����ĿΪNA | |
| B�� | 12gʯīϩ������ʯī���к�����Ԫ���ĸ���Ϊ0.5NA | |
| C�� | 235g����${\;}_{92}^{235}$U�����ѱ䷴Ӧ��${\;}_{92}^{235}$U+${\;}_{0}^{1}$n$\stackrel{�ѱ�}{��}$${\;}_{38}^{90}$Sr+${\;}_{54}^{136}$U+10${\;}_{0}^{1}$n�������������ӣ�${\;}_{0}^{1}$n����Ϊ10NA | |
| D�� | 1L 0.1mol•L-1��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1NA |
| A�� | ��NH4��2CO3��Һ�мӹ���NaOH��Һ�����ȣ�NH4++OH-=NH3•H2O | |
| B�� | ��������������������ҺAl+2OH-=AlO2-+H2�� | |
| C�� | ���ռ���Һ����������Cl2+OH-=Cl-+ClO-+H2O | |
| D�� | ̼��������Һ������������Һ��ϣ�HCO3-+OH-�TH2O+CO32- |
��̽��Ũ������ͭ��Ӧʱ��Ԫ������Ԫ�ص�������ǿ����ʵ��װ����ͼ��ʾ�����̶�װ������ȥ��
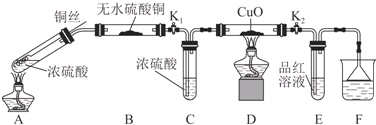
��1��A�з�Ӧ�Ļ�ѧ����ʽΪ2H2SO4��Ũ��+Cu$\frac{\underline{\;\;��\;\;}}{\;}$SO2��+SO42-+Cu2++2H2O��
��2��ʵ������У���֤��Ũ������ͭ��Ӧ���������Ԫ�ص�������ǿ����Ԫ�ص�������Dװ���к�ɫ������ɫ�ޱ仯��E����Һ��ɫ��
���о�������п��Ũ���ᷴӦ���������壺
��3��������п��Ũ���ᷴӦ��ȡ�Ķ������������п��ܺ��е�������������ˮ������
��4��ijͬѧ������װ�����ӳ�һ����ʵ��װ������֤��3�����ж��Ƿ���ȷ�������������������ʱ�����������ĸ�װ�õ��ܵı��������cd����dc��ab����ba��e����a��b����д����
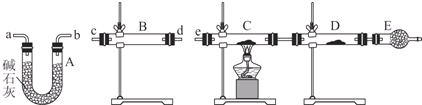
��5�����ݣ�4��ȷ����ʵ����̣��뽫ʵ��ʱ�й�װ������ʢҩƷ��ʵ�������ۻ�����������к����ϣ�
| װ�� | ��ʢҩƷ | ʵ������ | ���ۻ���� |
| B | �� | �� | �� |
| C | CuO���� | �� | �� |
�ڹ����ɰ�ɫ�����ɫ
��SO2�к���ˮ����
��Cװ���й����ɺ�ɫ��ɺ�ɫ��Dװ���ڹ����ɰ�ɫ�����ɫ
��SO2���������
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ⑪ | ⑫ |
 ����
����
��2���������ڣ���ϡ������Ԫ�أ���ԭ�Ӱ뾶����Ԫ���Ǣۣ�����ţ�������ͬ����ԭ�Ӱ뾶��С��Ԫ���Ǣࣨ����ţ�
��3��Ԫ�آ���Ԫ�آ���ȣ��ǽ����Խ�ǿ����Cl����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����b
a�������¢ߵĵ��ʺ͢�ĵ���״̬��ͬ
b������⻯��Ȣߵ��⻯���ȶ�
c��һ�������¢ߺ͢�ĵ��ʶ���������������Һ��Ӧ
��4��д��ʵ������ȡԪ�آٵ���̬�⻯����õĻ�ѧ����ʽCa��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O
��5��Ԫ�آٵ�����������Ӧ��ˮ�����Ũ��Һ�뵥��ͭ��Ӧ�Ļ�ѧ����ʽΪCu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O��
| A�� | ����Ϊͭ������Ϊ�����������ҺΪFeCl3��Һ | |
| B�� | ����Ϊ̼������Ϊ�����������ҺΪFe��NO3��3��Һ | |
| C�� | ����Ϊ��������Ϊ�����������ҺΪFe2��SO4��3��Һ | |
| D�� | ����Ϊ��������Ϊ�����������ҺΪCuSO4��Һ |
| A�� | 7.8gNa2O2��Na2S������к��е���������ĿΪ0.1NA | |
| B�� | pH=13������������Һ����K+��ĿԼΪ0.1NA | |
| C�� | ��״���£�5.6LO2��Ϊ������ʱת�Ƶĵ�����һ��ΪNA | |
| D�� | 18gD2O����������ĿΪ10NA |