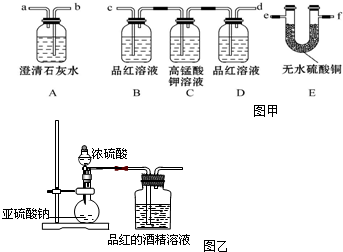
��Ŀ����
10��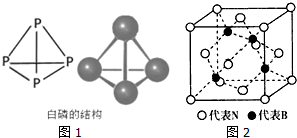 �������ڱ���ͬ��Ԫ�ص������ԣ���Ԥ��Ԫ�ص����ʣ�
�������ڱ���ͬ��Ԫ�ص������ԣ���Ԥ��Ԫ�ص����ʣ���1��PԪ�صĻ�̬ԭ����3��δ�ɶԵ��ӣ����ķ���ʽΪP4����ṹ��ͼ1��ʾ����ѧ��Ŀǰ�ϳ��� N4���ӣ�N ԭ�ӵ��ӻ����������sp3��N-N ���ļ���Ϊ60�㣻N4�ֽ���ܲ���N2���ͷų������������Ʋ�����;Ϊ�������ƽ�����ըҩ��
��2��N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��P��As��
��3��������������Ľṹ��ͼ2��ʾ��
�þ����У�Bԭ�������Nԭ�ӵ����������϶����ռ�ݴ����϶�ı���Ϊ50%����ٷ�������
��4��N��As��ͬ��Ԫ�أ�B��Ga��ͬ��Ԫ�أ������黯�ؾ�����������������ṹ���ƣ����־������۵�ϸߵ��ǵ����������黯�ؾ���ľ����߳�Ϊa pm�������ܶ�Ϊ$\frac{5.8��1{0}^{32}}{{N}_{A•}{a}^{3}}$g•cm-3 ���ú�a��ʽ�ӱ�ʾ����NAΪ�����ӵ�������ֵ����
���� ��1��PԪ��ԭ�Ӽ۵����Ų�ʽΪ3s22p3��N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�N4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��ÿ����Ϊ�������Σ�N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ��
��2��ͬ�������϶��µ�һ�����ܼ�С��
��3��Bԭ����Χ��4��Nԭ���γ���������ṹ��ÿ����������8����������������ṹ��ֻ��4�����Bԭ�ӣ�
��4�������黯�ؾ�����������������ṹ���ƣ�������ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ�Խǿ�������۵�Խ�ߣ����ݾ�̯�����㾧����As��Gaԭ����Ŀ����ʾ���������������ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺��1��PԪ��ԭ�Ӽ۵����Ų�ʽΪ3s22p3����̬ԭ����3��δ�ɶԵ��ӣ�N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�N4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���ÿ����Ϊ�������Σ�N-N ���ļ���Ϊ60�㣻N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ��
�ʴ�Ϊ��3��sp3��60�㣻�������ƽ�����ըҩ��
��2��ͬ�������϶��µ�һ�����ܼ�С���ʵ�һ�����ܣ�N��P��As��
�ʴ�Ϊ��N��P��As��
��3��Bԭ����Χ��4��Nԭ���γ���������ṹ��ÿ����������8����������������ṹ��ֻ��4�����Bԭ�ӣ�Bԭ��ռ�ݴ����϶�ı���Ϊ50%��
�ʴ�Ϊ���������壻50%��
��4�������黯�ؾ�����������������ṹ���ƣ�������ԭ�Ӿ��壬ԭ�Ӱ뾶N��As��B��Ga���ʵ������й��ۼ���ǿ��������ľ����۵���ߣ�������As��Gaԭ����Ŀ��Ϊ4����������Ϊ4��$\frac{145}{{N}_{A}}$g�������ܶ�Ϊ4��$\frac{145}{{N}_{A}}$g�£�a��10-10 cm��3=$\frac{5.8��1{0}^{32}}{{N}_{A•}{a}^{3}}$g��cm-3��
�ʴ�Ϊ��������$\frac{5.8��1{0}^{32}}{{N}_{A•}{a}^{3}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ�жϡ����ӽṹ�������ܡ������ṹ����㡢�۷е�Ƚϵȣ���3��Ϊ�״��㡢�ѵ㣬��Ҫѧ���߱�һ���Ŀռ�����ע��ͬ����Ԫ�ص�һ�������쳣�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �������м���һ������ϡ���� | |
| B�� | ��MgSO4��H2SO4�Ļ��Һ�е������Ba��OH��2��Һ | |
| C�� | ��NaOH��Һ��ͨ��һ����CO2���� | |
| D�� | �����ʵ���Ũ��֮��Ϊ2��7��AlCl3��NaOH��Һ�������� |
| A�� | ������Һ | B�� | ���� | C�� | ���� | D�� | ��������Һ |
| A�� | ��1 L 1 mol/L ��NaCl��Һ��ȡ��10 mL����Ũ������1 mol/L | |
| B�� | �Ƴ�0.5 L 10 mol/L �����ᣬ��Ҫ�Ȼ������� 112L����״���� | |
| C�� | 0.5 L 2 mol/L BaCl2 ��Һ�У�Ba2+ ��Cl- ����λ3��6.02��1023 | |
| D�� | 10 g 98% ���� ���ܶ�Ϊ 1.84g/cm3����10 mL 18.4 mol/L �����Ũ���Dz�ͬ�� |
�±���25��ʱijЩ�ε�Ũ�Ȼ�������
| ��ѧʽ | CaSO4 | Ag2SO4 | PbSO4 |
| Ksp | 4.9��10-5 | 1.2��10-5 | 1.6��10-8 |
A���ձ� B����Ͳ C������ƿ D����ƿ E��������
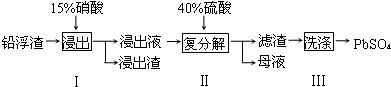
��2������I��NO����������Һ�к������Ľ���������Ϊ Pb2+��д��Pb�μӷ�Ӧ�Ļ�ѧ����ʽ
3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O��Ϊ��ֹAg���ܽ������Һ������I����ʱӦע����������������ʹPb����ʣ�࣮
��3��ĸҺ��ѭ�������ڲ���I����������Ҫ��HNO3����һ�����ʻ�ѧʽ������ĸҺ�в����� SO42-���࣬ѭ������ʱ���ܳ��ֵ������ǽ���ʱ����Pb2+����PbSO4��������ų�������PbSO4�IJ��ʣ�
��4����PbSO4 ��Ʒ���е�����������ƣ�����Pb��NO3��2��Һ���ϴ�ӣ��Եõ�������PbSO4��
��5��Ǧ���طŵ�ʱ�����ĵ缫��ӦʽΪPbO2+2e-+4H++SO42-=PbSO4+2H2O�������Ǧ��������Դ��ⱥ��ʳ��ˮ��ȡCl2����֪ijǦ������������Һ�����Ϊ0.80L�����ǰ���ḢҺŨ��Ϊ4.50mol•L-1�����Ƶ�4.48LCl2ʱ���ڱ�״���£����������ϵ�������������Һ��Ũ��Ϊ��������ǰ��������Һ��������䣩4 mol•L-1��
| A�� | MgCl2�����ڣ� $\frac{\underline{\;���\;}}{\;}$ Mg+Cl2�� | B�� | Al2O3+3C $\frac{\underline{\;2125��\;}}{\;}$2Al+3CO�� | ||
| C�� | Fe2O3+3CO $\frac{\underline{\;����\;}}{\;}$2Fe+3CO2 | D�� | HgS+O2$\frac{\underline{\;����\;}}{\;}$ Hg+SO2 |
| A�� | 22.4LCH4��CH3Cl�Ļ���������еķ�����ĿΪNA | |
| B�� | 1mol������������й��õ��Ӷ���Ϊ3NA | |
| C�� | �ö��Ե缫���1 LŨ�Ⱦ�Ϊ2 mol/L��AgNO3��Cu��NO3��2�Ļ����Һ������0.2 NA������ת��ʱ����������6.4g���� | |
| D�� | 0.1mol Na2CO3•10H2O�ھ����л���Һ�У����е�CO32-���Ӿ�С��0.1 NA |
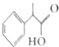 ��ʾ��
��ʾ��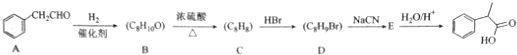
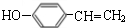 ��
��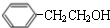 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$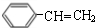 +H2O��C��D�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
+H2O��C��D�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��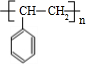 ��
��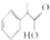 ����������ȷ����B������ĸ��ţ�
����������ȷ����B������ĸ��ţ�