��Ŀ����
9����֪��������Ũ��Ϊ0.1mol/L��������Һ��pH�����| ���� | NaF | Na2CO3 | NaClO | NaHCO3 |
| pH | 7.5 | 11.6 | 9.7 | 8.3 |
| A�� | ����ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��H2CO3��HClO��HF | |
| B�� | ����CO2ͨ��0.1mol/L Na2CO3��Һ����Һ���ԣ�����Һ�У�2c��CO32-��+c��HCO3-��=0.1mol/L | |
| C�� | ����������ʵ���Ũ�ȵ�NaClO��Һ��NaF��Һ������������С��Nǰ��N�� | |
| D�� | ��Na2CO3��Һ��ͨ��������HF���壬��ѧ��Ӧ����ʽΪ��Na2CO3+2HF�TCO2+H2O+2NaF |
���� A����ҺpHԽ��Ӧ�������Խ����Ũ�����ʱ����Һ�ĵ�����Խǿ��
B����ҺΪ����ʱ��c��H+��=c��OH-�������ݵ���غ��֪��2c��CO32-��+c��HCO3-��=c��Na+����
C�����������ж�ˮ��̶ȣ�Ȼ���ϵ���غ��ж�����Һ�к�����������Ŀ��С��
D��HF��������Ӧ���ɵ���NaHCO3��
��� �⣺A����ҺpHԽ��Ӧ�������Խ�������ݱ������ݿ�֪�����Դ�СΪ��HF��H2CO3��HClO��HCO3-���¶ȡ�Ũ����ͬʱ������Խǿ����Һ��������Խǿ����������Һ�����Դ�СΪ��HClO��H2CO3��HF����A����
B������CO2ͨ��0.1mol/L Na2CO3��Һ����Һ���ԣ���c��H+��=c��OH-�������ݵ���غ��֪��2c��CO32-��+c��HCO3-��=c��Na+��=0.2mol/L����B����
C��NaClO��NaF��Һ�д���������ӡ������ӷ���ˮ��ʹ��Һ�ʼ��ԣ�������Ũ��С��10-7mol/L����������HF��HClO������������ˮ��̶ȴ��ڷ����ӣ��ʴ���������Һ������������Ũ�ȴ��ڷ����ƣ�����������Һ�е�������Ũ��С�ڷ����ƣ�����Һ��������Ũ����ȣ�����Һ��������Ũ��=c��Na+��+c��H+����������Һ�����ȣ������������Һ����������ĿС�ڷ����ƣ�����Nǰ��N������C��ȷ��
D����Na2CO3��Һ��ͨ��������HF���壬��Ӧ����̼�����ƺͷ����ƣ���ȷ�Ļ�ѧ��Ӧ����ʽΪ��Na2CO3+HF�TNaHCO3+NaF����D����
��ѡC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ж���Һ����ǿ��Ϊ���ؼ���ע�����յ���غ㡢�����غ㼰�ε�ˮ��ԭ���ĺ��弰Ӧ�÷���������������ѧ���ķ������������Ӧ��������

| A�� | ��ij�ܱ������м���0.5molN2��1.5molH2����ַ�Ӧ�����������ʺ��е�N-H����Ϊ3NA | |
| B�� | ��״���£�2.24LSO3���е�ԭ����Ϊ0.4 NA | |
| C�� | 71g��������������Ӧ������ת�Ƶĵ�����Ϊ2NA | |
| D�� | ��ⱥ��ʳ��ˮʱ������������22.4LH2ʱ����·��ת�Ƶĵ�����Ϊ2NA |
| A�� | ����NaClO����ˮ�е�NH3������N2��3ClO-+2NH3�TN2��+3Cl-+3H2O | |
| B�� | ��ϡHNO3�еμ�Na2SO3��Һ��SO32-+2H+�TSO2��+H2O | |
| C�� | NaAlO2��Һ��AlO2-��ˮ�⣺AlO2-+2H2O�TAl��OH��3��+OH- | |
| D�� | ��Na2SiO3��Һ��ͨ�����SO2��SiO32-+SO2+H2O�TH2SiO3��+SO32- |
| A�� | 78g��Na2S��Na2O2��ɵĹ���������е���������ΪNA | |
| B�� | ��״���£�22.4L CCl4�к��еķ�����ΪNA | |
| C�� | 1mol Cu�ֱ���������Cl2��S��Ӧ��ת�Ƶ�������Ϊ2NA | |
| D�� | 1mol•L-1 FeCl3��Һ�к��е�Fe3+��ĿС��NA |
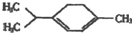
| A�� | 5�� | B�� | 6�� | C�� | 7�� | D�� | 8�� |
| A�� | ú��������Һ���������仯��ú�ĸ����ǻ�ѧ�仯 | |
| B�� | �Ҵ����������⡢�������Ƶ�����Һ�����Խ������������ﵽ������Ŀ�� | |
| C�� | ����ȼ�ϵ�ط��磬������ֱ���ڿ�����ȼ�շ�������ת���ʸ� | |
| D�� | �ϳɰ���ӦN2��g��+3H2��g��?2NH3��g�� OH��O����������ܼӿ췴Ӧ���ʲ����ת���� |
| A�� | �ֱ����Na0H��Һ�����ȣ�����ع��ͣ����������ͣ��Ϳ����ͣ����͡�ú�͵ȣ� | |
| B�� | Ϊ����������Һ�е�Cl-��SO42-���ȼ���������Һ����ȥ����������ᱵ��Һ | |
| C�� | Ϊ̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ��ʱ���Ƚ����������������������Һ��Ϻ�����ˮԡ���� | |
| D�� | ������FeBr2��FeI2�Ļ����Һ��ͨ������������Ȼ�����Һ���ɡ����գ��õ�FeCl3���� |
| A�� | �����о�����ǦԪ�� | B�� | �����в���Ϊ���Ӻ�ǦԪ�ص����� | ||
| C�� | �����к�ǦԪ����һ��ָ�귶Χ�� | D�� | ���϶�����ȷ |


 ���ɵĽṹ��ʽ
���ɵĽṹ��ʽ


 ����дһ�֣�
����дһ�֣�