��Ŀ����
7����Na2CO3•10H2O��������0.08mol•L-1��Na2CO3��Һ500mL����Ҫ��ش��������⣺��1����������ƽ��ҩ�ס��ձ����������⣬��ȱ�ٵı�Ҫ����Ϊ��ͷ�ιܡ�500mL����ƿ����������ƽ�����ľ�������Ϊ11.4g��
��2�������в�����д�������������ܽ⡢��ȴ�����ת��ʱ�����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�Σ�����ϴ��Һȫ��ת�Ƶ�����ƿ�У�
��3���ж����в�����������Һ��Ũ��ƫ�ߵ���BCE��
A������ʱ��������ƿ�̶���
B������ʱ��������ƿ�̶���
C�����ܽ�δ����ȴ����Һֱ��ת������ƿ��ͽ��ж��ݲ���
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
E������ǰNa2CO3•10H2O������ʧȥ�˲��ֽᾧˮ
F��ת��ǰ����ƿδ���и��ƿ������������ˮ������
���� ��1������ʵ������IJ�����ȷ����Һ������������������m=CVM������Ҫ���ʵ�������
��2��������Һ��ϴ�ӵ���ȷ�������
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1����Һ���Ʋ��������У��������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡҩƷ�����ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�500ml����ƿ�У����ò�����������ϴ�Ӳ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ����Ի���Ҫ������Ϊ��500mL����ƿ����ͷ�ιܣ�
Na2CO3•10H2O��������0.08mol•L-1��Na2CO3��Һ500mL����ҪNa2CO3•10H2O������m=0.08mol/L��286g/mol��0.5L=11.4g��
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�11.4g��
��2����Һʱ����Ϊ����ƿƿ����ϸ��Ӧ���ò������������������ݺ����ϴ�ӣ�����������ˮϴ���ձ���������2��3�Σ�����ϴ��Һȫ��ת�Ƶ�����ƿ�У�
�ʴ�Ϊ���������ձ�����������
��3��A������ʱ��������ƿ�̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���A��ѡ��
B������ʱ��������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���Bѡ��
C�����ܽ�δ����ȴ����Һֱ��ת������ƿ��ͽ��ж��ݲ�������ȴ����Һ���ƫС��������ҺŨ��ƫ�ߣ���Cѡ��
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���������Һ���ƫ����ҺŨ��ƫ�ͣ���D��ѡ��
E������ǰNa2CO3•10H2O������ʧȥ�˲��ֽᾧˮ���������������ʵ���ƫ����ҺŨ��ƫ�ߣ���Eѡ��
F��ת��ǰ����ƿδ���и��ƿ������������ˮ�����������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��F��ѡ��
��ѡ��BCE��
���� ���⿼��һ�����ʵ���Ũ����Һ�����Ʋ������������ȣ���ȷ����ԭ�������������ǽ���ؼ���ע������c=$\frac{n}{V}$�����������ķ�����ע������ʹ��ע�����

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�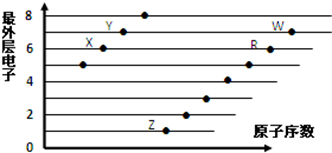
| A�� | X��R��ͬһ���� | |
| B�� | ԭ�Ӱ뾶��W��R��X | |
| C�� | ��̬�⻯������ԣ�X��Y | |
| D�� | X��Z�γɵĻ��������������Ӹ�����Ϊ1��2 |
| �� ���� | I A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | J | F | H |
��2������������ˮ���������ǿ����NaOH��������ǿ����HClO4�������Ե���Al��OH��3 ��
��3����B��C��D��G�У�ԭ�Ӱ뾶�ɴ�С˳�����Na��Al��C��F��
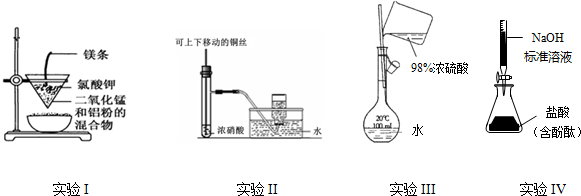
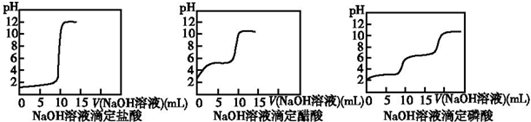
| A�� | ʵ��I���Ʊ������� | |
| B�� | ʵ��II���Ʊ����ռ�NO2 | |
| C�� | ʵ��III������һ�������ʵ���Ũ�ȵ�ϡ������Һ | |
| D�� | ʵ��IV���ⶨδ֪�����Ũ�� |
| A�� | 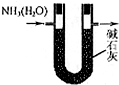 ����ͼװ�ø��ﰱ�� | |
| B�� | 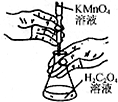 ����ͼװ�ý��и��������Һ�ζ�������Һʵ�� | |
| C�� | 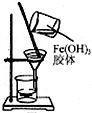 ����ͼװ�ÿɷ�����������������Fe��OH��3��ˮ | |
| D�� |  ����ͼװ�ÿ���֤���ԣ����̼����� |