��Ŀ����
�ȵ���ԭ���Ļ����۵��ǣ�ԭ������ͬ�Ҽ۵���������ȵķ��ӻ����Ӿ�����ͬ�Ļ�ѧ�����ͺͿռ乹�ͣ�����Ϊ�ȵ����壮�ȵ�����Ľṹ���ƣ���������������磺N2��CO��C22-��CN-Ϊ�ȵ����壮
��1����֪CaC2Ϊ���ӻ������CaC2�ĵ���ʽΪ ��
��2���۱�ϩ���׳�������ë���ɱ�ϩ�����CH2=CH-CN���ۺϷ�Ӧ���ɣ���CH2=CH-CN��Cԭ�ӵ��ӻ���ʽΪ �������ЦҼ��ͦм���֮��Ϊ ��
��3��CO������ɽ���ԭ��M�γ������M��CO��n��������������ԭ�Ӽ۵���������λ���ṩ��������֮��Ϊ18����MΪFe����n= ��
��4��CO��N2�Ľṹ���ƣ������к��й����������ɱ�ʾΪC��O���±������ߵļ������ݣ���λ��kJ?mol-1��
CO��N2�л�ѧ���ʽϻ��õ��� ���������˵��ԭ�� ��
��5��Fe3+��Fe2+��Co3+��Co2+������CN- �γ���������������Һ�м������KCN��Һ����������ɫ����K4[Fe��CN��6]������������Һ����ͨ���������������ɫ����K3[Fe��CN��6]����K4[Fe��CN��6]��K3[Fe��CN��6]�����ж������ڵ������������� �������ţ�
A�����Ӽ����� B�����ۼ����� C�������� D����λ������ E�����»���
��6��д����NO3- ��Ϊ�ȵ�����ķ��� ��д��һ�֣���
��1����֪CaC2Ϊ���ӻ������CaC2�ĵ���ʽΪ
��2���۱�ϩ���׳�������ë���ɱ�ϩ�����CH2=CH-CN���ۺϷ�Ӧ���ɣ���CH2=CH-CN��Cԭ�ӵ��ӻ���ʽΪ
��3��CO������ɽ���ԭ��M�γ������M��CO��n��������������ԭ�Ӽ۵���������λ���ṩ��������֮��Ϊ18����MΪFe����n=
��4��CO��N2�Ľṹ���ƣ������к��й����������ɱ�ʾΪC��O���±������ߵļ������ݣ���λ��kJ?mol-1��
| C-O | C=O | C��O | |
| CO | 357.7 | 798.9 | 1071.9 |
| N-N | N=N | N��N | |
| N2 | 154.8 | 418.4 | 941.7 |
��5��Fe3+��Fe2+��Co3+��Co2+������CN- �γ���������������Һ�м������KCN��Һ����������ɫ����K4[Fe��CN��6]������������Һ����ͨ���������������ɫ����K3[Fe��CN��6]����K4[Fe��CN��6]��K3[Fe��CN��6]�����ж������ڵ�������������
A�����Ӽ����� B�����ۼ����� C�������� D����λ������ E�����»���
��6��д����NO3- ��Ϊ�ȵ�����ķ���
���㣺���ȵ���ԭ������Ӧ��,���ۼ����γɼ����ۼ�����Ҫ����,�����ijɼ����,ԭ�ӹ���ӻ���ʽ���ӻ������ж�,��ͬ����Ľṹ��������������������
ר�⣺��ѧ���뾧��ṹ
��������1�����ݵȵ�����ṹ���ƣ�д��C22-�ĵ���ʽ��Ȼ��������ӻ��������ʽ����д����д������ʽ��
��2�������ӻ������=�Ҽ���Ŀ+�¶Ե��Ӷ�����ȷ���ӻ����������1��������1���Ҽ���һ��˫������1���Ҽ���1���м���һ����������1���Ҽ���2���м���
��3�������Fe��CO��x������ԭ������ԭ�ӣ���۵�������8��ÿ�������ṩ�ĵ�������2���ݴ��ж�xֵ��
��4�������ṩ�ļ��ܼ���������еĵ�һ���м��ļ��ܣ�����Խ��Խ�ȶ���
��5�������������K4[Fe��CN��6]��K3[Fe��CN��6]���������Ӽ������ۼ�����λ����
��6�����ݵȵ�����Ķ��壬ֻҪԭ����Ŀ�ͼ۵���������ͬ���ǵȵ����壻
��2�������ӻ������=�Ҽ���Ŀ+�¶Ե��Ӷ�����ȷ���ӻ����������1��������1���Ҽ���һ��˫������1���Ҽ���1���м���һ����������1���Ҽ���2���м���
��3�������Fe��CO��x������ԭ������ԭ�ӣ���۵�������8��ÿ�������ṩ�ĵ�������2���ݴ��ж�xֵ��
��4�������ṩ�ļ��ܼ���������еĵ�һ���м��ļ��ܣ�����Խ��Խ�ȶ���
��5�������������K4[Fe��CN��6]��K3[Fe��CN��6]���������Ӽ������ۼ�����λ����
��6�����ݵȵ�����Ķ��壬ֻҪԭ����Ŀ�ͼ۵���������ͬ���ǵȵ����壻
���
�⣺��1��N2��C22-Ϊ�ȵ����壬C22-�ĵ���ʽΪ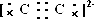 ��CaC2�ɸ����Ӻ�C22-���ɣ�����ʽΪ��
��CaC2�ɸ����Ӻ�C22-���ɣ�����ʽΪ��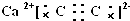 ��
��
�ʴ�Ϊ��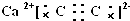 ��
��
��2����ϩ�����CH2=CH-CN��˫���ϵ�̼ԭ���γ�3���Ҽ����µ��Ӷԣ�sp2�ӻ����뵪������̼ԭ���γ�2���Ҽ����µ��Ӷԣ�sp�ӻ���CH2=CH-CN�����к���6���Ҽ���3���м�����Ŀ֮��Ϊ 2��1��
�ʴ�Ϊ��sp2�ӻ���sp�ӻ���2��1��
��3�������Fe��CO��x������ԭ������ԭ�ӣ���۵�������8��ÿ�������ṩ�ĵ�������2��8+2x=18��x=5��
�ʴ�Ϊ��5��
��4�����ݵ������ӽṹ֪��һ��CO�����к���2���м������ݱ��м���֪��CO�е�һ���м��ļ�����1071.9kJ/mol-798.9kJ/mol=273kJ/mol��N2�е�һ���м��ļ�����941.7kJ/mol-418.4kJ/mol=523.3kJ/mol��CO�е�һ���м��ļ��ܽ�С������CO�ĵ�һ���м���N2�����ϣ�����һ����̼�ȵ������ã�
�ʴ�Ϊ��CO��CO�е�һ���м��ļ�����1071.9kJ/mol-798.9kJ/mol=273kJ/mol��N2�е�һ���м��ļ�����941.7kJ/mol-418.4kJ/mol=523.3kJ/mol������һ���м������������CO��N2С�ܶ࣬��CO�����ã�
��5���������K4[Fe��CN��6]��K3[Fe��CN��6]���������Ӽ������ۼ�����λ�������������������»�����
�ʴ�Ϊ��C��E��
��6�����ݵȵ�����Ķ��壬NO3-�ĵȵ������������ԭ�ӷ��ӻ����ӣ��Ҽ۵�����������Ϊ�ȵ�����ķ���SO3��
�ʴ�Ϊ��SO3��
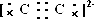 ��CaC2�ɸ����Ӻ�C22-���ɣ�����ʽΪ��
��CaC2�ɸ����Ӻ�C22-���ɣ�����ʽΪ��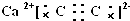 ��
���ʴ�Ϊ��
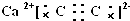 ��
����2����ϩ�����CH2=CH-CN��˫���ϵ�̼ԭ���γ�3���Ҽ����µ��Ӷԣ�sp2�ӻ����뵪������̼ԭ���γ�2���Ҽ����µ��Ӷԣ�sp�ӻ���CH2=CH-CN�����к���6���Ҽ���3���м�����Ŀ֮��Ϊ 2��1��
�ʴ�Ϊ��sp2�ӻ���sp�ӻ���2��1��
��3�������Fe��CO��x������ԭ������ԭ�ӣ���۵�������8��ÿ�������ṩ�ĵ�������2��8+2x=18��x=5��
�ʴ�Ϊ��5��
��4�����ݵ������ӽṹ֪��һ��CO�����к���2���м������ݱ��м���֪��CO�е�һ���м��ļ�����1071.9kJ/mol-798.9kJ/mol=273kJ/mol��N2�е�һ���м��ļ�����941.7kJ/mol-418.4kJ/mol=523.3kJ/mol��CO�е�һ���м��ļ��ܽ�С������CO�ĵ�һ���м���N2�����ϣ�����һ����̼�ȵ������ã�
�ʴ�Ϊ��CO��CO�е�һ���м��ļ�����1071.9kJ/mol-798.9kJ/mol=273kJ/mol��N2�е�һ���м��ļ�����941.7kJ/mol-418.4kJ/mol=523.3kJ/mol������һ���м������������CO��N2С�ܶ࣬��CO�����ã�
��5���������K4[Fe��CN��6]��K3[Fe��CN��6]���������Ӽ������ۼ�����λ�������������������»�����
�ʴ�Ϊ��C��E��
��6�����ݵȵ�����Ķ��壬NO3-�ĵȵ������������ԭ�ӷ��ӻ����ӣ��Ҽ۵�����������Ϊ�ȵ�����ķ���SO3��
�ʴ�Ϊ��SO3��
���������⿼���˵���ʽ����д���ȵ���ԭ������ѧ�����ܵļ��㡢ԭ���ӻ��ȣ���Ŀ�ۺ��Խ�ǿ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���л�������������������ε�һ���ǣ�������
| A��Na2CO3��KOH��CaO |
| B��CO��NaOH��KCl |
| C��H2O��H2SO4��NaCl |
| D��CO2��KOH��FeO |
����ʵ�鲻����Ϊ�ж����ݵ��ǣ�������
| A���ƺͼطֱ�����ˮ��Ӧ���жϽ������ǿ�� |
| B��Br2��I2�ֱ���������H2��Ӧ���ж������ķǽ������ǿ�� |
| C��̼������Һ�Լ��ԣ���������Һ�����ԣ��ж�����̼�ķǽ������ǿ�� |
| D����MgSO4��Al��NO��3��Һ�зֱ��������İ�ˮ���ж�þ�����Ľ������ǿ�� |




 ���ɣ���ṹ��ʽ��
���ɣ���ṹ��ʽ��