��Ŀ����
3��Ӧ����ͼװ�ã���Ӳ���������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɡ�������Fe��ˮ�����ķ�Ӧʵ�顱��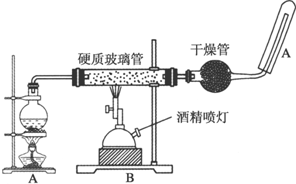
����ɸ�ʵ���е����⣺
��1��д���÷�Ӧ�Ļ�ѧ����ʽ��3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2����������ԭ��Ӧ����������H2O��
��2��ʵ��ǰ���������װ�ý��еIJ����Ǽ���װ�õ������ԣ�
��3��Բ����ƿ��ʢװ����ˮ����װ�����Ⱥ����Ҫ�������ṩ�������ϵ�ˮ������
��ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ�������Ƿ�ֹ���У�
��4��������Һ���Ƿ���������������KSCN��Һ��д��ѧʽ������������Һ��ΪѪ��ɫ��
���� A��Բ����ƿ�ڼ��������¿��ṩˮ������B�ڼ��������£�����ˮ������Ӧ����������������������������������Թ����������ſշ����ռ�������������KSCN���������ӣ������ܶȱȿ���С������KSCN���������ӣ��Դ˽����⣮
��� �⣺��1��Fe��ˮ������Ӧ�����������������������÷�ӦΪ��FeԪ�صĻ��ϼ����ߣ�FeΪ��ԭ�ԣ�HԪ�صĻ��ϼ۽��ͣ���ˮΪ��������
�ʴ�Ϊ��3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��H2O��
��2��ʵ��ǰ���������װ�ý��������Լ�飬�ʴ�Ϊ������װ�õ������ԣ�
��3����Ϊ��Ӧ��Ϊˮ��������������������ȵ�Ŀ�ľ����ṩˮ���������Ƭ�ɷ�ֹ���з������ʴ�Ϊ���ṩ�������ϵ�ˮ��������ֹ���У�
��4�����������ӣ��ɼ���KSCN��������Ϸ�Ӧ����Һ��ΪѪ��ɫ���ʴ�Ϊ��KSCN����Һ��ΪѪ��ɫ��
���� ���⿼���������仯���������ʵ�飬��Ŀ�ѶȲ���ע����������ˮ������Ӧԭ����������ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ�����������

��ϰ��ϵ�д�
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
�����Ŀ
1������������ȷ���ǣ�NAΪ�����ӵ�������ֵ����������
| A�� | 7.8gNa2O2���еĹ��ۼ���Ϊ0.2NA | |
| B�� | 7.8gNa2S��Na2O2�Ļ�������������Ϊ0.3NA | |
| C�� | ��״���£�22.4L�嵥�ʺ���NA������� | |
| D�� | 0.2 molNa����ȫ��������7.8gNa2O2��ת�Ƶ��ӵ���ĿΪ0.4NA |
12��������ĿҪ�ش��������⣺
��ijͬѧ���Ҵ��������Ũ������ȡ����������װ����ͼ1��ʾ��
��1��װ���и���ܵ������Ƿ�ֹС�Թ���Һ�巢������
��2��С�Թ��е��Լ�����˳��ΪB
A��Ũ���� �Ҵ� ���� B���Ҵ� Ũ���� ���� C������ Ũ���� �Ҵ�
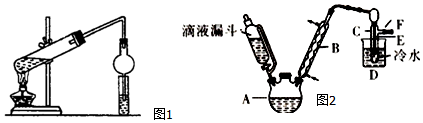
II����֪��R-OH+HX��R-X+H2O��ͼ2��ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ�Ʊ��������װ�ã�ͼ��ʡȥ�˼���װ�ã��Ҵ��������顢���йز������ݼ�����
��3����ʵ����Ӧ��ȡ�ļ��ȷ�ʽ��ˮԡ���ȣ�
��4��Ϊ��ȥ��Ʒ�е�Br2�����ѡ��������Һ��ϴ�Ӳ�ƷB��
A���������� B���������� C���⻯��
��ijͬѧ���Ҵ��������Ũ������ȡ����������װ����ͼ1��ʾ��
��1��װ���и���ܵ������Ƿ�ֹС�Թ���Һ�巢������
��2��С�Թ��е��Լ�����˳��ΪB
A��Ũ���� �Ҵ� ���� B���Ҵ� Ũ���� ���� C������ Ũ���� �Ҵ�
II����֪��R-OH+HX��R-X+H2O��ͼ2��ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ�Ʊ��������װ�ã�ͼ��ʡȥ�˼���װ�ã��Ҵ��������顢���йز������ݼ�����
| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | �����ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
��4��Ϊ��ȥ��Ʒ�е�Br2�����ѡ��������Һ��ϴ�Ӳ�ƷB��
A���������� B���������� C���⻯��

 ����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ��������գ�
����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ��������գ� ��ʵ������������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��ʵ������������ͼ��ʾ��װ����ȡ�����������ش��������⣺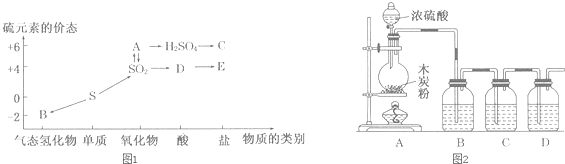
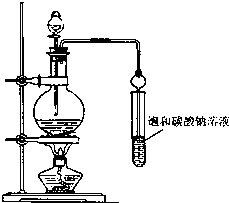 ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������ŨH2SO4��ϣ�Ȼ��Һ©���ߵμӴ��ᣬ��������ֱ���ռ������Ʒ�ɵõ������Ҵ������ѡ����ᡢ����ˮ�����������ֲ�Ʒ���ݴ˻ش����⣺
ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������ŨH2SO4��ϣ�Ȼ��Һ©���ߵμӴ��ᣬ��������ֱ���ռ������Ʒ�ɵõ������Ҵ������ѡ����ᡢ����ˮ�����������ֲ�Ʒ���ݴ˻ش����⣺ CH3COOC2H5+H2O��
CH3COOC2H5+H2O��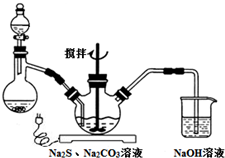 NaCNΪ�綾���ij��ȤС������ϵ�֪��ʵ�������NaCN��Һ����Na2S2O3��Һ���нⶾ���٣����ǿ�չ����������ʵ�飬�����Ҫ��ش����⣺
NaCNΪ�綾���ij��ȤС������ϵ�֪��ʵ�������NaCN��Һ����Na2S2O3��Һ���нⶾ���٣����ǿ�չ����������ʵ�飬�����Ҫ��ش����⣺