��Ŀ����
7��NaCl��Ʒ�к�������CaCl2��Na2SO4�Ͳ�����ˮ�����ʣ�Ϊ���ᴿNaCl��ijͬѧ��������²������̣�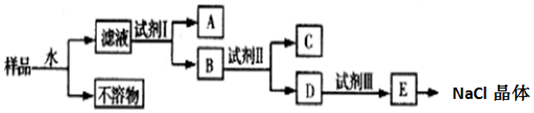
��ش��������⣺
��1���Լ�I�Ļ�ѧʽΪBa��OH��2��BaCl2���������dz�ȥSO42-�������ӷ��ţ�
��2�������Լ���ʱ������Ӧ�Ļ�ѧ����ʽΪBaCl2+Na2CO3=BaCO3��+2NaCl��Ba��OH��2+Na2CO3=BaCO3��+2NaOH��CaCl2+Na2CO3=CaCO3��+2NaCl��
��3���Լ�������������ᣮ���������������Լ�������������������Լ����Ѿ�����������ѡ��BC������ĸ����
A��AgNO3��Һ B��pH��ֽ C��Na2CO3��Һ
��4�����ϲ�����������3�������漰���˲�������E�л��NaCl����IJ��������������ᾧ�������ִ�������ʱ��Ӧ��ֹͣ���ȣ�
���� �����̿�֪���ܽ����������Ϊ��������˿ɷ��룬��Һ�м��Լ�IΪBa��OH��2��BaCl2���ɳ�ȥNa2SO4��AΪBaSO4��B�к�CaCl2��Ba��OH��2��BaCl2���ټ��Լ���ΪNa2CO3��CΪCaCO3��BaCO3��D�к�NaCl��Na2CO3�����Լ���Ϊ���ᣬEΪNaCl��Һ�������ᾧ�õ�NaCl���Դ������
��� �⣺��1��������������֪���Լ�IΪBa��OH��2��BaCl2������Ϊ��ȥSO42-���ʴ�Ϊ��Ba��OH��2��BaCl2��SO42-��
��2�������Լ���ʱ������Ӧ�Ļ�ѧ����ʽΪBaCl2+Na2CO3=BaCO3��+2NaCl ��Ba��OH��2+Na2CO3=BaCO3��+2NaOH��CaCl2+Na2CO3=CaCO3��+2NaCl��
�ʴ�Ϊ��BaCl2+Na2CO3=BaCO3��+2NaCl ��Ba��OH��2+Na2CO3=BaCO3��+2NaOH��CaCl2+Na2CO3=CaCO3��+2NaCl��
��3��������������֪���Լ���Ϊ���ᣬ���������Ƿ������ѡ��pH��ֽ�ⶨpH���̼���ƹ۲��Ƿ������ݣ���NaCl��HCl������������Ӧ���ɳ���������ѡ��������
�ʴ�Ϊ�����BC��
��4�����������У���ˮ���Լ�I���Լ��������˷��룬��3�������漰���˲�������E�л��NaCl����IJ��������������ᾧ�������ִ�������ʱֹͣ���ȣ��������ȼ��ȣ�
�ʴ�Ϊ��3�������ᾧ�����ִ������壮
���� ���⿼����������ᴿ��Ϊ��Ƶ���㣬���������з����ķ�Ӧ���������뷽��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��Ԫ�ػ�����֪ʶ��ʵ��Ľ�ϣ���Ŀ�ѶȲ���

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�| A�� | ��̼������Һ�еμ��Ȼ�����Һ��������ɫ���� | |
| B�� | ���з�̪������������Һ�еμ����ᣬ��ɫ��ȥ | |
| C�� | ���������Һ��ͨ�������̼��������ɫ��״���� | |
| D�� | ����ͭ��Һ�в�����Ƭ����Ƭ�ϳ��ֺ�ɫ���� |
| A�� | ͬ��ͬѹ�£���ͬ���������嶼ռ����ͬ����� | |
| B�� | ͬ��ͬѹ�£���ͬ��������嶼������ͬ��Ŀ�ķ��� | |
| C�� | �ڱ���£�1Ħ���κ����ʵ������ԼΪ22.4�� | |
| D�� | 1Ħ��ij��������ԼΪ22.4����������������״��һ���DZ���� |
| A�� | Сֽ���е����ʿɳ��ڳ������������ | |
| B�� | ��ʯ���������� | |
| C�� | �˸����Ϊ����� | |
| D�� | ���и�Ԫ�صĴ�����̬Ϊ����̬ |
| A�� | ����0.2mol K2SO4 | |
| B�� | K+�����ʵ���Ũ��Ϊ0.2mol•L-1 | |
| C�� | K�����ʵ���Ϊ0.8mol | |
| D�� | ȡ��1LK2SO4��Һ��ʣ��K2SO4��Һ��Ũ�ȱ�Ϊ0.1mol•L-1 |