��Ŀ����
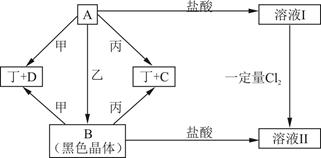
��15�֣���������Ԫ�أ��ֱ�λ��Ԫ�����ڱ���ǰ�ĸ���ͬ���ڣ���ԭ�������ܺ�Ϊ48.�����ǿ���ɼס��ҡ����������ֵ��ʺ�A��B��C��D���ֻ�������мס���Ϊ�ǽ������ʣ�������Ϊ��������.��Щ���ʼ��ת����ϵ����ͼ��ʾ����Ӧ������ʡ�ԣ�
��ش��������⣺
��1����ɶ���Ԫ�������ڱ��е�λ��________________.B������_____________��C���ʵ���;֮һ_____________________.
��2��д��A+�� B�Ļ�ѧ����ʽ___________________________________.
B�Ļ�ѧ����ʽ___________________________________.
��3���ڼ��������£�������̬D�ɷ�����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________.
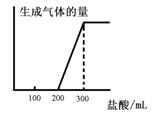
��4����21.6g A���������ҷ�Ӧ����B��A��B������ͼʾת��Ϊ��ҺI����ҺII�����������Ӧ��ǡ�ý�����ȫ��������ҺI��ͨ��________mol Cl2������ַ�Ӧ��ǡ��ʹ���ʵ��������ҺII��ȫ��ͬ.

��ش��������⣺
��1����ɶ���Ԫ�������ڱ��е�λ��________________.B������_____________��C���ʵ���;֮һ_____________________.
��2���A+��
 B�Ļ�ѧ����ʽ___________________________________.
B�Ļ�ѧ����ʽ___________________________________.��3���ڼ��������£�������̬D�ɷ�����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________.
��4����21.6g A���������ҷ�Ӧ����B��A��B������ͼʾת��Ϊ��ҺI����ҺII�����������Ӧ��ǡ�ý�����ȫ��������ҺI��ͨ��________mol Cl2������ַ�Ӧ��ǡ��ʹ���ʵ��������ҺII��ȫ��ͬ.
��15�֣���1����������VIII�壨2�֣�����������������2�֣�
�ͻ���ϻ���������ԭ�ϣ�2�֣�
��2��6FeO+O2 2Fe3O4��3�֣�
2Fe3O4��3�֣�
��3��3Fe+4H2O(g) Fe3O4+4H2��3�֣�
Fe3O4+4H2��3�֣�
��4��0.1��3�֣�
�ͻ���ϻ���������ԭ�ϣ�2�֣�
��2��6FeO+O2
 2Fe3O4��3�֣�
2Fe3O4��3�֣���3��3Fe+4H2O(g)
 Fe3O4+4H2��3�֣�
Fe3O4+4H2��3�֣���4��0.1��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ



 _______��
_______�� ��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________��
��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________�� ��Ӧ��������ȥ)��
��Ӧ��������ȥ)��


 6����Ϊ��������Ҫ�ɷ�֮һ���ĵ���ʽΪ ���ҵĿռ乹��Ϊ �����ϡ��Һ�ܸ��������۷�Ӧ�������ӷ�Ӧ����ʽΪ ��
6����Ϊ��������Ҫ�ɷ�֮һ���ĵ���ʽΪ ���ҵĿռ乹��Ϊ �����ϡ��Һ�ܸ��������۷�Ӧ�������ӷ�Ӧ����ʽΪ ��
 ��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��