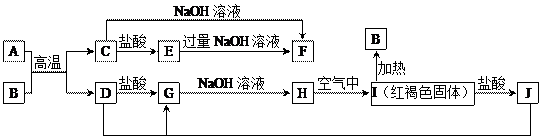��Ŀ����
��12�֣�Mg���仯������Է�������ת�������ַ�Ӧ��������ˮ����ȥ������֪X��Y��ZΪ��̬���ʣ�B������ΪҺ̬��������D����ɫ��ӦΪ��ɫ��C��G���ð���̲�����A�����E��ˮ��Һ������ij�ֹ�ҵ������

��1��д���������ʵĻ�ѧʽ
A Y
��2��д��C�ĵ���ʽ
��3��д��MgCl2��D��Ӧ�Ļ�ѧ����ʽ
��4������ˮ��ƽ�����۽���A+B+Mg��C+X+ MgCl2��ԭ��_______________________��
��5����0.1molCO2ͨ��1L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ������ ��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��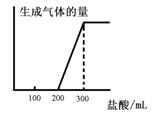

��1��д���������ʵĻ�ѧʽ
A Y
��2��д��C�ĵ���ʽ
��3��д��MgCl2��D��Ӧ�Ļ�ѧ����ʽ
��4������ˮ��ƽ�����۽���A+B+Mg��C+X+ MgCl2��ԭ��_______________________��
��5����0.1molCO2ͨ��1L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ������
 ��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
��

��ϰ��ϵ�д�
�����Ŀ
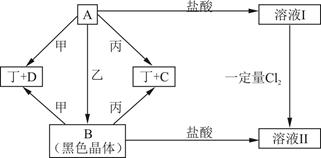
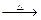 B�Ļ�ѧ����ʽ___________________________________.
B�Ļ�ѧ����ʽ___________________________________.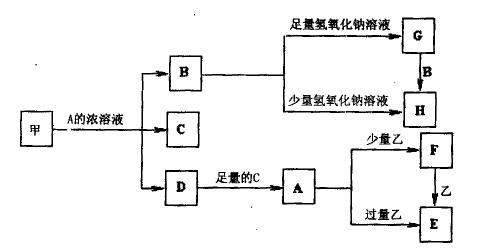
 ������Ӳ�����2����Z��ͬ���ڵ�����Ԫ����ԭ�Ӱ뾶���W�ǵؿ��к������Ľ���Ԫ�أ�L�ĵ��ʾ����۵�ߡ�Ӳ�ȴ���һ����Ҫ�İ뵼����ϡ��û�ѧ����ش��������⣺
������Ӳ�����2����Z��ͬ���ڵ�����Ԫ����ԭ�Ӱ뾶���W�ǵؿ��к������Ľ���Ԫ�أ�L�ĵ��ʾ����۵�ߡ�Ӳ�ȴ���һ����Ҫ�İ뵼����ϡ��û�ѧ����ش��������⣺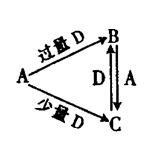
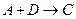 ���Ȼ�ѧ����ʽ��
���Ȼ�ѧ����ʽ��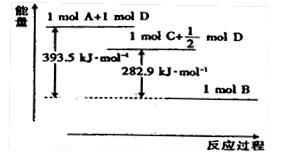
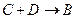 ��
��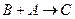 �����ӷ���ʽΪ_________
�����ӷ���ʽΪ_________ _______________________��
_______________________��
 ���ɵ�ˮ��������������ȥ��
���ɵ�ˮ��������������ȥ��