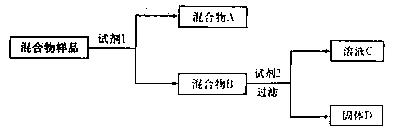
��Ŀ����
��9�֣��ɶ�����Ԫ����ɵ�X��Y��Z��M����ѧ��ѧ�������������ʣ���������ͼ��ʾ��ת����ϵ�����������ش����⡣

��1����X��Y��Z����ͬ�ֳ�������Ԫ�أ�M��O2��XΪ___________��Z��������һ�������»�������һ�ֲ��д���÷�Ӧ�Ļ�ѧ����ʽ________��
��2����X��Y��Z����ͬ�ֳ����ǽ���Ԫ�أ�����Z��ʹƷ����Һ��ɫ��
��X�ĵ���ʽΪ__________________ _______��
_______��
��Z�����������������Һ��Ӧ�����ӷ���ʽ�ǣ�_____________________________��
��3����X��ˮ��Һ�Լ��ԣ�X��M ��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________��
��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________��

��1����X��Y��Z����ͬ�ֳ�������Ԫ�أ�M��O2��XΪ___________��Z��������һ�������»�������һ�ֲ��д���÷�Ӧ�Ļ�ѧ����ʽ________��
��2����X��Y��Z����ͬ�ֳ����ǽ���Ԫ�أ�����Z��ʹƷ����Һ��ɫ��
��X�ĵ���ʽΪ__________________
 _______��
_______����Z�����������������Һ��Ӧ�����ӷ���ʽ�ǣ�_____________________________��
��3����X��ˮ��Һ�Լ��ԣ�X��M
 ��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________��
��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________����9�֣�
��1��Na���ƣ���1�֣� Na2O2+H2=2NaOH��2�֣�
��2����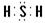 ��2�֣� ��SO2+2OH-=SO32-+H2O��2�֣�
��2�֣� ��SO2+2OH-=SO32-+H2O��2�֣�
��3��(NH4)2CO3+CO2+H2O=2NH4HCO3��(NH4)2S+H2S =2NH4HS
(NH4)2SO3+SO2+H2O=2NH4HSO3��2�֣�
��1��Na���ƣ���1�֣� Na2O2+H2=2NaOH��2�֣�
��2����
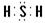 ��2�֣� ��SO2+2OH-=SO32-+H2O��2�֣�
��2�֣� ��SO2+2OH-=SO32-+H2O��2�֣���3��(NH4)2CO3+CO2+H2O=2NH4HCO3��(NH4)2S+H2S =2NH4HS
(NH4)2SO3+SO2+H2O=2NH4HSO3��2�֣�
��

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
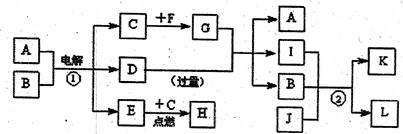
 ��
��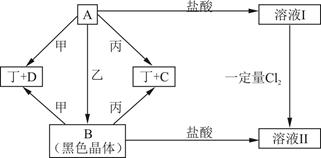
 B�Ļ�ѧ����ʽ___________________________________.
B�Ļ�ѧ����ʽ___________________________________. д����һ�����뷽��ʽ�� ��
д����һ�����뷽��ʽ�� �� ��֪ÿ1mol�ײμӷ�Ӧ�� 4 mol����ת�ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��֪ÿ1mol�ײμӷ�Ӧ�� 4 mol����ת�ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ�� �� 2YX3(g)��һ�������´ﵽƽ�⡣
2YX3(g)��һ�������´ﵽƽ�⡣