��Ŀ����
8���±���Ԫ�����ڱ���һ���֣�������ĸ�ֱ����һ��Ԫ�أ�
��1��mԪ�������ڱ��е�λ���ǵ������ڢ�A�壮
��2�������й�˵����ȷ����ABC������ĸ����
A��b��c��dԪ�صķǽ�����������
B��f��g��hԪ�ص�ԭ�Ӱ뾶��С
C��md2��bd2�Ļ�ѧ�������ƣ�������������
D��e��n����ۺ����������ǿ����e��n
E��a��f�ֱ���d��ɵĻ�������������ѧ��������ȫ��ͬ
F���ñ���ֻ��4��Ԫ����ɵĵ��ʾ��е�����
��3��a��c��n��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�������NH4Cl��
���� ���ݸ�Ԫ�������ڱ��е����λ�ÿ�֪��aΪH��bΪC��cΪN��dΪO��eΪF��fΪNa��gΪMg��hΪAl��mΪS��nΪCl��pΪCaԪ�أ����Ԫ��������֪ʶ���н��
��� �⣺����ͼʾ��֪��aΪH��bΪC��cΪN��dΪO��eΪF��fΪNa��gΪMg��hΪAl��mΪS��nΪCl��pΪCaԪ�أ�
��1��mΪSԪ�أ�ԭ������Ϊ16������㺬��6�����ӣ�λ�����ڱ��е������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��2��A��b��c��dλ��ͬһ���ڣ���ԭ����������������Ԫ�صķǽ�����������A��ȷ��
B��f��g��hλ��ͬһ���ڣ�ԭ������Խ��ԭ�Ӱ뾶ԽС��������Ԫ�ص�ԭ�Ӱ뾶��С����B��ȷ��
C��md2��bd2�ֱ�ΪSO2��NO2�����߶��ܹ������Һ��Ӧ��������һ���������ԣ��������ƵĻ�ѧ���ʣ���C��ȷ��
D��e��n�ֱ�ΪF��ClԪ�أ��ǽ�����F��Cl������F���������ۣ�������ۺ����ᣬ��D����
E��a��f��d�ֱ�ΪH��Na��OԪ�أ�H��Na�γɵĻ�����ΪNaH��Na��O�γɵ�Ԫ��Ϊ�������ơ������ƣ�NaH��������Ϊ���ӻ�������ǹ��������л����й��ۼ�����E����
F���ñ���Ԫ����ɵĵ��ʾ��е����Ե���C��Na��Mg��Al��Ca5��Ԫ�أ���F����
�ʴ�Ϊ��ABC����
��3��a��c��n�ֱ�ΪH��N��Cl��H��N��Cl��ԭ�Ӹ�����Ϊ4��1��1���ɵĻ�����ΪNH4Cl��
�ʴ�Ϊ��NH4Cl��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷԭ�ӽṹ��Ԫ�����ڱ��Ĺ�ϵΪ���ؼ���ע������Ԫ�����ڱ��ṹ��Ԫ�����������ݣ�����������ѧ���ķ������������Ӧ��������

| A�� | �����ĵ���ʽ�� | B�� | ��������ӵĽṹʽ��H-O-Cl | ||
| C�� | Na��ԭ�ӽṹʾ��ͼ�� | D�� | �����ӵĵ���ʽ��Ca2+ |
| A��Ŀ�ģ�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� | B��Ŀ�ģ�����������Һ |
���� 1mol/L 1mL���ᡡ��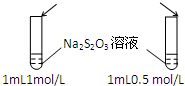 | 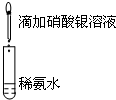 ���� ���� |
| C��Ŀ�ģ��Ƚ�Al��Fe��Cu��� | D��Ŀ�ģ�ʵ������ȡ���� |
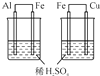 |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
 �ݱ����������ض�����һ������£������˴�����ġ���ȼ����������Ҫ�Ǽ����ˮ�γɵ�ˮ���CH4•nH2O����
�ݱ����������ض�����һ������£������˴�����ġ���ȼ����������Ҫ�Ǽ����ˮ�γɵ�ˮ���CH4•nH2O������1���ڳ��³�ѹ�£�����ȼ�����ᷢ���ֽⷴӦ���仯ѧ����ʽ��CH4•nH2O=CH4��+nH2O��
��2��������Ƴɺϳ�����CO��H2�������Ƴɼ״����������湩Ӧ���ŵ�ȼ�ͣ�
����101KPaʱ��1.6g CH4��g����H2O��g����Ӧ����CO��H2������20.64kJ���������H2O��g����Ӧ���Ȼ�ѧ����ʽ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+206.4 kJ•mol-1��
��CH4����ȫȼ��Ҳ���Ƶúϳ�����CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����
��H=-35.4kJ•mol-1�����ԭ��ѡ�����Դ���ýǶȣ��ȽϷ����ٺ͢ڣ��ϳɼ״������˷���Ϊ�ڣ�����ţ���ԭ����ѡ��CH4����ȫȼ�գ��ƺϳ�����ʱ���ų�������ͬʱ�õ�CO��H2Ϊ1��2����ǡ����ȫ��Ӧ�ϳɼ״���
��3�����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ��������ºϳɼ״�����������Ҫ��Ӧ���£�
��CO��g��+2H2��g��=CH3OH��g����H1
��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H2
��CO2��g��+H2��g��=CO��g��+H2O��g����H3
�ش��������⣺
��֪��Ӧ������صĻ�ѧ�������������£�
| ��ѧ�� | H-H | C-O | C��O | H-O | C-H |
| E/��kJ•mol-1�� | 436 | 343 | 1 076 | 465 | 413 |
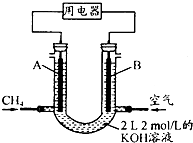
��4����ȼ����CH4��������;�ǣ���CH4��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ��ʾ��ͼ��A��BΪ�����̼����������ͨ�˼��飬�ڱ�״���£����ļ������VL��
��O��V��44.8Lʱ������ܷ�Ӧ����ʽΪCH4+2O2+2KOH=K2CO3+3H2O��
��44.8L��V��89.6Lʱ�������缫��ӦΪCH4-8e-+9CO32-+3H2O=10HCO3-��
| A�� | K | B�� | Ca | C�� | I | D�� | Ne |
 ũ��ʦ�������ʻ�ʩ����S-�տ��ؼ������ӽṹ��ͼ������ʹ�ʻ���ʱʢ����
ũ��ʦ�������ʻ�ʩ����S-�տ��ؼ������ӽṹ��ͼ������ʹ�ʻ���ʱʢ����



 ����Ӧ����Ϊ�ӳɷ�Ӧ��
����Ӧ����Ϊ�ӳɷ�Ӧ�� ��
��


