��Ŀ����
12��ij��ȤС�����Ʊ���������֤��һϵ�����ʣ�ʵ��װ����ͼ��ʾ��ʡ�Լг�װ�ã�����֪����������ƣ�Na2S2O3����Һ�ڹ�ҵ�Ͽ���Ϊ���ȼ�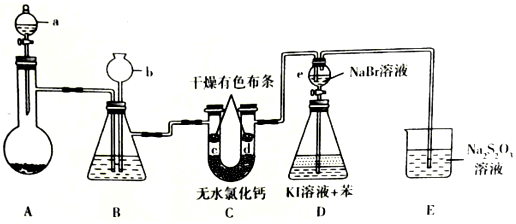
�ش��������⣺
��1������a�������Ƿ�Һ©��������ʢ��Ũ���ᣬ��ƿA ��ʢ�еĹ����Լ���KMnO4����KClO3���ȣ����ѧʽ����
��2��װ��B��ʢ�б���NaCl��Һ��װ��B��������acd��
a����ȥC12�е�����HCl b������ c���ṩ����ˮ���� d���۲�װ���Ƿ����
��3��c����ɫ������ɫ����d������ɫ����˵��Cl2��Ư�����ã�HC1O��Ư�����ã�
��4��ʵ�������e �Ļ�����ʹ���е���Һ���˵���ƿD�У�ҡ����ƿ�����ú�ɹ۲쵽�ϲ���ҺΪ�Ϻ�ɫ��
��5��װ��E�з�����Ӧ�����ӷ���ʽΪS2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+��
���� ��ͼ��װ�ÿ�֪��װ��A�з���KMnO4����KClO3���ȣ�����Һ©���з���Ũ���ᣬ����������ͨ�뱥��ʳ��ˮ��ȥ���е��Ȼ������壬Ȼ��ͨ��C���������ɫ������������״��������ɹ۲쵽c��������ɫ��d������ɫ������ͨ��©��D�У���Һ���ɫ���ٵ���KI��Һ�뱽�ķֲ��Һ���У��������ɫ��������������������������Һ���գ�Na2S2O3������+2�ۣ�����������SO42-���Դ������
��1����������a�������Լ��������������Ʒ������װ��A���������ȡ��������ѡ���ڳ����������Ȼ������������
��2�����ɵ������л���HCl��װ��B�������г�ȥ�����е��Ȼ��⣬�ṩ����ˮ������������ȫƿ��
��3����ʪ����������Ư���ԣ�������������߱�Ư���ԣ�������Ư�����õ��Ǵ����
��4��������ȡ��ˮ�е��嵥�ʣ������ܶȱ�ˮС��
��5��Na2S2O3������+2�ۣ�����������SO42-�����ݵ�ʧ�����غ��ԭ���غ���д��
��� �⣺��1������a���в���������©����Ϊ��Һ©��������ʢ��Ũ���ᣬ��ƿA ���ڳ����������Ȼ�����ȡ��������ѡ��KMnO4����KClO3���ȣ�����ӦΪ��2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O��KClO3+6HCl��Ũ��=KCl+3Cl2��+3H2O��
�ʴ�Ϊ����Һ©����KMnO4����KClO3���ȣ���
��2�����������ڱ���ʳ��ˮ����HCl��������ˮ�����ñ���ʳ��ˮ��ȥ�������������Ȼ������壬����ͨ��C���������ɫ������������״��������ɹ۲쵽c��������ɫ��d������ɫ��װ��B�ṩ����ˮ��������������ʱB�еģ�ѹǿ����B�г���©����Һ���������γ�ˮ�����ɹ۲�װ���Ƿ����������acd���ϣ�
�ʴ�Ϊ��acd��
��3����װ��c��ͨ��������d������ɫ��������ɫ����Ϊ������������߱�Ư���ԣ�c������ɫ������ɫ����˵������������ҺB�������ˮ����ʪ����������Ư���ԣ�������Ư�����õ���������ˮ��Ӧ���ɵĴ��������Ư���ԣ�
�ʴ�Ϊ��Cl2��Ư�����ã�HC1O��Ư�����ã�
��4��ʵ�������e �Ļ�����ʹ���е���Һ���˵���ƿD�У���ˮ�м��뱽��ʳȺ췢����ȡ�������ܶȱ�ˮС���ϲ���ҺΪ�Ϻ�ɫ��
�ʴ�Ϊ���ϲ���ҺΪ�Ϻ�ɫ��
��5��Na2S2O3������+2�ۣ�����������SO42-����װ��E����Ӧ�����ӷ�Ӧ����ʽ��S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+��
�ʴ�Ϊ��S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+��
���� ���⿼���������Ʊ�ʵ�鼰����ʵ�飬Ϊ��Ƶ���㣬����ʵ��װ�õ����á����ʵ����ʼ����ӵķ���Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д�| A�� | NaOH�ͽ����� | B�� | NaOH ��CO2 | C�� | Na2CO3��HCl | D�� | Na��O2 |
| A�� | ��������Ȼ������Ҫ�ɷ֣��ܷ���ȡ����Ӧ�������ܷ���������Ӧ | |
| B�� | ʵ�����У����ý����Ƽ����Ҵ����Ƿ���ˮ | |
| C�� | ֻ��ˮ�����𱽡���������Ȼ�̼ | |
| D�� | ֲ���Ͳ���������ȡ��ˮ�е��� |

| A�� | ͼ2 �з�Ӧ���������������� | |
| B�� | ͼ1 �������ҵķ�Ӧ����ʹ���˴��� | |
| C�� | ͼl ��������0��5 minʱ����v${\;}_{��{N}_{2}��}$=0.012mol/��L•min�� | |
| D�� | ͼ1 ���������ڷ�Ӧ��ƽ�ⳣ��Ϊ2.5 |
| A�� | �ҹ��Ŵ��Ĵ���֮һ�ڻ�ҩ�����ᡢ�����ľ̿��һ����������Ƴ� | |
| B�� | PH�Ʋ�����������к͵ζ��յ���ж� | |
| C�� | ʯ�͵ķ���ú�ĸ���������Һ�����������仯 | |
| D�� | ����պŨ������ް��������Ͱ����Ĺܵ��Ƿ�©�� |
| ���� | ���� |
| ����ʢ��4.0gNa2O2���ձ��м���50mL����ˮ | ���ҷ�Ӧ��������������ʹ������ľ����ȼ������ȫ���ܽ�õ�����ɫ��Һa |
| ������Һa�е������η�̪ | ��Һ��죬10���Ӻ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
| ������Һ�м�������MnO2��ĩ | ���д������ݲ���������������Ҳ��ʹ������ľ����ȼ |
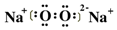 �������ԣ�ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ�Ӧ��ͬλ��ʾ��ԭ�����Ա�
�������ԣ�ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ�Ӧ��ͬλ��ʾ��ԭ�����Ա�ʾ��Ӧ�Ļ�����д��Na218O2��H2O��Ӧ�Ļ�ѧ����ʽ2Na218O2+2H2O�T2Na18OH+2NaOH+18O2����
��2���������к�ɫ��ȥ�Ŀ���ԭ������Һa�й���H2O2���̪������Ӧ��
��3���÷�Ӧ2MnO4-+5H2O2+6H+=2Mn2++502��+8H2O�ⶨ��Һa��H2O2������ȡ20.00mL��Һa����ϡH2SO4���ѧʽ���ữ����0.002mol•L-1KMnO4��Һ�ζ������յ�ʱƽ������10.00mLKMnO4��Һ���ζ�ʱKMnO4��ҺӦװ���ᣨ�����ʽ�ζ����У��յ�ȷ���ķ����ǵ������һ��ʱ��Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ����������Һa��c��H2O2��=0.0025mol•L-1
��4������Һa�еμ�FeSO4��Һ��������Ӧ�����ӷ���ʽΪ4H2O2+4Fe2++6H2O=O2��+4Fe��OH��3��+8Na+��
��5����FeSO4��Һ�м���һ����Na202���壬�������ʵ���Ϊ2��1������Ӧ����Ӧ�����������ɣ�д��
��Ӧ�����ӷ���ʽ3Na2O2+6 Fe2++6H2O=6Na++4Fe��OH��3��+2Fe3+��
| A�� | 78g���к��е�̼̼˫������ĿΪ3NA | |
| B�� | 16g��Cu2S��CuO��ɵĻ�����к��е���������Ϊ0.2NA | |
| C�� | ��1molH2��1molI2����һ�ܱ������г�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| D�� | 1mo1Fe��������Ũ���Ṳ�ȷ�Ӧ������SO2�ķ�����ΪNA |

����˵����ȷ���ǣ�������
| A�� | �ܽ�����ѡ�������ᣬ���Դӳ�����ǿ����ѡ������һ�֣��Լ�Xѡ������ | |
| B�� | ����1��һ������SiO2������pH��Ϊ��ʹAl3+ת��ΪAl��OH��3���������2 | |
| C�� | ����Һ2�õ�FeSO4•7H2O��Ʒ�Ĺ����У�ֻ��Ҫ������������Һ�����������壬ֹͣ���������������ɼ��� | |
| D�� | ���ı䷽��������Һ1��ֱ�Ӽ�NaOH���������õ��ij����������ܽ⣬����Һ���ᾧ����Ҳ�ɵõ�FeSO4•7H2O |