��Ŀ����
5��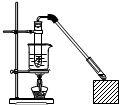 ������Ҵ���Ӧ��װ����ͼ��ʾ�����Թ������3mL�Ҵ���Ȼ��һ��ҡ����һ�������ؼ���2mLŨ�����2mL�����ᣬ�þƾ���С�ľ��ȵؼ���10min����������������������ͨ�뵽����̼������Һ��Һ���ϣ�
������Ҵ���Ӧ��װ����ͼ��ʾ�����Թ������3mL�Ҵ���Ȼ��һ��ҡ����һ�������ؼ���2mLŨ�����2mL�����ᣬ�þƾ���С�ľ��ȵؼ���10min����������������������ͨ�뵽����̼������Һ��Һ���ϣ���1������̼������Һ���������к����ᡢ�ܽ��Ҵ������������������ܽ�ȣ�
��2��ʵ��װ���г����ܵ������ǵ�����������
��3�������ܲ�����Һ�����µ�Ŀ���Ƿ�ֹ������
��4��ʵ���в�ȡ�ļ��ȷ�ʽ��ˮԡ���ȣ����ּ��ȷ�ʽ�ĺô���Ҫ�б��ڿ����¶ȣ�ʹ���Ⱦ��ȣ�
��5�����÷�Һ©���ᴿ�Թ��е������������ڲ���ʱҪע����ϴ�Ӻ��÷�Һǰ����������������������c�����ţ������������÷֣���
a�������������ڼ��ӻ���Ȧ�ϴ��ϲ����� b�������������ڼ��ӻ���Ȧ�ϴ���
c�������ĵ�ס�����ϲ����ӵ��ú���� d����������ƽ����ʵ��̨�ϴ���
�����Ƶõ���Ӧ�Ӹ÷�Һ©����b�����ţ������������÷֣���
a���²����� b���Ͽڵ��� c�������ԣ�
���� ��1������̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻
��2�����ȷ�Ӧ�������Ҵ�������ӷ��������������������ã�
��3�����ݵ�������Һ���¿��ܷ�������������
��4��ˮԡ���ȣ����ڿ����¶ȣ�ʹ���Һ���Ⱦ��ȣ�
��5���پ��÷�Һǰ����������ؼ�Ҫ��ֹҺ��������
�������������ܶȱ�ˮС��������ˮ��
��� �⣺��1���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ���к����ᡢ�ܽ��Ҵ������������������ܽ�ȣ�
��2����֪���������ķе�Ϊ77�棻�Ҵ��ķе�Ϊ78.5�棻����ķе�Ϊ117.9�棬���ȷ�Ӧ�������Ҵ��������ӷ��������������������ã�ͬʱ�䵼�����ã�
�ʴ�Ϊ��������������
��3�����ܲ��ܲ�����Һ�У�����Ҫ���ڱ���̼������Һ��Һ���ϣ�����Һ���¿��ܷ����������ʴ�Ϊ����ֹ������
��4���ӷ�Ӧװ�ÿ������ȷ���Ϊˮԡ���ȣ�ˮԡ���ȱ��ڿ����¶ȣ���ˮ���Ȱ�Χ�Թܿ���ʹ���Һ���Ⱦ��ȣ��ʴ�Ϊ��ˮԡ���ȣ����ڿ����¶ȣ�ʹ���Ⱦ��ȣ�
��5���پ��÷�Һǰ����������ؼ�Ҫ��ֹҺ�����������Ծ�����������������ĵ�ס�����ϲ����ӵ��ú��������ѡ��c��
���������������ܶȱ�ˮС���������������ϲ㣬��Һ©�����ϲ�Һ����Ͽڵ�������ѡ��b��
���� ���⿼�������������Ʊ�����Ŀ�ѶȲ���ע��ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ���������������ѧ���������������������Ӧ����ѧ֪ʶ���ʵ�������������

 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д� ͼ��ʵ����������������װ�ã�
ͼ��ʵ����������������װ�ã���1������ǰ��ͨ����Ҫ���Թܢ��м������Ƭ��Ŀ���Ƿ�ֹ���У�
��2��Ϊ��֤��Ũ��������ã�ijͬѧ����������4��ʵ�飬ʵ���¼�����
| ʵ�� ��� | �Թܢ����Լ� | �Թܢ����Լ� | �л�����/cm |
| A | 3mL�Ҵ���2mL���ᡢ1mL18mol•L-1Ũ���� | ����Na2CO3��Һ | 5.0 |
| B | 3mL�Ҵ���2mL���� | ����Na2CO3��Һ | 0.1 |
| C | 3mL�Ҵ���2mL���ᡢ6mL3mol•L-1���� | ����Na2CO3��Һ | 1.2 |
| D | 3mL�Ҵ���2mL���ᡢ6mL6mol•L-1���� | ����Na2CO3��Һ | 1.2 |
�ڷ���ʵ��A��B��C�����ݣ����Եó�Ũ�����ڷ�Ӧ�е������Ǵ�������ˮ����ʵ��D��ʵ��C���գ������ܵó��Ľ����ǶԸ÷�Ӧ������õ�ʵ����ΪH+��
��3������Na2CO3��Һ���������к����ᡢ�ܽ��Ҵ�����������ˮ�е��ܽ�ȣ����ֲ㣮
| A�� | A�ǹ��壬C�����壬����Ӧ���� | B�� | A�����壬C�����壬����Ӧ���� | ||
| C�� | A�����壬C�ǹ��壬����Ӧ���� | D�� | A�����壬C�����壬����Ӧ���� |
| A�� | ����ʱ�����ɽ�����Һ���ձ���ֱ�ӵ���©���� | |
| B�� | ��������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� | |
| C�� | ��Һ����ʱ���ֽ���Һ©�����²�Һ����¿ڷų����ٽ��ϲ�Һ����¿ڷų� | |
| D�� | ֻҪ��Һ������Ϳ��������������� |

 ������ѧ֪ʶ������������⣺
������ѧ֪ʶ������������⣺
 ��1�������dzµ��㡱������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ�����Ҵ��������Ũ��������ȡ�����������ش��������⣺
��1�������dzµ��㡱������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ�����Ҵ��������Ũ��������ȡ�����������ش��������⣺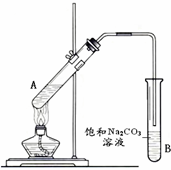 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺