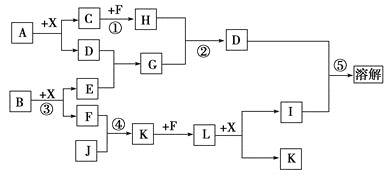
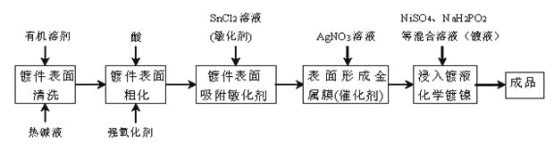
��Ŀ����
����Ŀ��������X������Ԫ����ɣ�Ϊ̽������ɵ����ʣ���Ʋ��������ʵ�飺
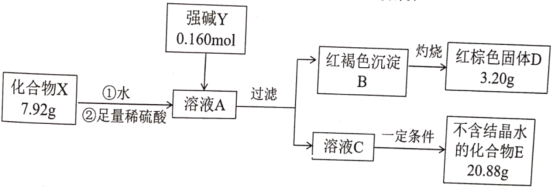
��ʾ��������E����ɫ��ӦΪ��ɫ������ɫ�ܲ�����
��ش�
��1��X�Ļ�ѧʽ��________________��ǿ��Y�ĵ���ʽΪ________________��
��2���ڳ��º���������£�������X�����ȶ����ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������һ�����嵥�ʡ�
�ٻ�����X��ˮ��Ӧ�����ӷ���ʽΪ________________��
��������Ի�����X���ȶ��Խ����˴������о�����ȡ����һ���Ľ�չ���������ʿ����������X��ˮ��Һ���ȶ��Ե���________________��
A KHSO4 B K2CO3 C CH3COOK D K2SO3
��Ϊ�о��¶ȶԻ�����Xˮ��Һ�ȶ��Ե�Ӱ�죬�����һ��ʵ�鷽����________________________________________________��
��3��������X�ж����Ʊ���������һ�ַ�������ǿ��Y�������ô����������ɫ����B��Ӧ���仯ѧ����ʽΪ________________��
���𰸡�K2FeO4 ![]() 4 FeO42-+10H2O=4Fe(OH)3��+3O2��+8OH- BC ȡ����������������ֱ����������Թ��в��ӵ�����ˮ�ܽ⣬Ȼ��һ֧�Թ�������ˮ�У���һ֧�Թ�������ˮ�У��۲���������Ŀ����� 3KClO+4KOH+2 Fe(OH)3=2 K2FeO4+3KCl+5H2O
4 FeO42-+10H2O=4Fe(OH)3��+3O2��+8OH- BC ȡ����������������ֱ����������Թ��в��ӵ�����ˮ�ܽ⣬Ȼ��һ֧�Թ�������ˮ�У���һ֧�Թ�������ˮ�У��۲���������Ŀ����� 3KClO+4KOH+2 Fe(OH)3=2 K2FeO4+3KCl+5H2O
��������
���������֪��������E����ɫ��ӦΪ��ɫ������ɫ�ܲ���������˵���û�����E�к�KԪ�أ���������˼ά��������X��ˮ�����ᷴӦ�õ�����ҺA�м���ǿ�����Ӧ�����ĺ��ɫ�ij���ΪFe(OH)3����֤��X�к���Ԫ�أ�3.20g����ɫ����DΪFe2O3����n(Fe2O3)= ![]() =
=![]() =0.02mol����n(Fe) =0.04mol����Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ������K2SO4������Ϊ20.88 g��n(K2SO4)=
=0.02mol����n(Fe) =0.04mol����Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ������K2SO4������Ϊ20.88 g��n(K2SO4)=![]() =0.12mol������ԭ�����к��е�K+�����ʵ���Ϊ0.12mol��2-0.16mol=0.08mol����˿��Ƴ�����X�л�����KԪ�أ�����һ��Ԫ��ӦΪ��Ԫ�أ�������m(O)= 7.92g -0.04mol��56g/mol- 0.08mol�� 39g/mol= 2.56g������n(O)=
=0.12mol������ԭ�����к��е�K+�����ʵ���Ϊ0.12mol��2-0.16mol=0.08mol����˿��Ƴ�����X�л�����KԪ�أ�����һ��Ԫ��ӦΪ��Ԫ�أ�������m(O)= 7.92g -0.04mol��56g/mol- 0.08mol�� 39g/mol= 2.56g������n(O)=![]() =0.16mol������ڻ�����A�и�Ԫ��ԭ�ӵĸ�����ΪK��Fe:O=0.08��0.04��0.16=2:1:4���ʻ�ѧʽΪK2FeO4���ݴ˷�������
=0.16mol������ڻ�����A�и�Ԫ��ԭ�ӵĸ�����ΪK��Fe:O=0.08��0.04��0.16=2:1:4���ʻ�ѧʽΪK2FeO4���ݴ˷�������
��������������֪��
��1��X������Ԫ�طֱ�ΪK��Fe��O����ԭ�Ӹ�����Ϊ2:1:4���ʻ�ѧʽΪK2FeO4��ǿ��YΪ�������أ��ɼ����������������ӹ��ɣ������ʽΪ��![]() ���ʴ�Ϊ��K2FeO4��
���ʴ�Ϊ��K2FeO4��![]() ��
��
��2���ڳ��º���������£�������X�����ȶ����ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ����Ϊ�������������������ԭ��Ӧ�Ĺ�����֪�����������嵥��ӦΪ��������
�ٸ��������ˮ��Ӧ�����ӷ���ʽΪ��4FeO42-+10H2O=4Fe(OH)3��+3O2��+8OH-��
�ڸ���������Ӧ��֪��Ҫ������ȶ��ԣ�Ӧ��ʹ��Һ�Լ��ԣ���
A. KHSO4��ˮ�е���������ӣ�ʹ��Һ�����ԣ����������ȶ��ԣ�
B. K2CO3ˮ���Լ��ԣ��������⣻
C. CH3COOKˮ���Լ��ԣ��������⣻
D. K2SO3��ˮ���Լ��ԣ������л�ԭ�ԣ�����K2FeO4����������ԭ��Ӧ�������棬���������ȶ��ԣ�
��ѡBC��
�۹̶������������ı��¶ȣ������������Ŀ����������ʵ��Ϊȡ����������������ֱ����������Թ��в��ӵ�����ˮ�ܽ⣬Ȼ��һ֧�Թ�������ˮ�У���һ֧�Թ�������ˮ�У��۲���������Ŀ�����
��3����KOH�����������ô�������������������Ʊ�������صĻ�ѧ����ʽ3KClO+4KOH+ 2Fe(OH)3= 2K2FeO4+3KCl+5H2O��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�����Ŀ������ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni(OH)2��̼�ۡ���������Ϳ�����������Ƴɡ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ���������£�
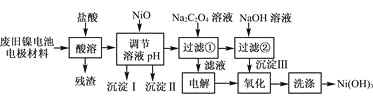
��֪����NiCl2������ˮ��Fe3����������Ni2����
����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O4��2H2O
��ij�¶���һЩ�������������Ksp����������������pH���±���ʾ��
|
| ��ʼ����pH | ������ȫpH |
|
|
|
|
|
|
|
|
|
|
|
|
�ش��������⣺
(1) ��NiO������Һ��pH����������������________�ͳ�����__________(�ѧʽ)��
(2) д������Na2C2O4��Һ�ķ�Ӧ�Ļ�ѧ����ʽ��_____________________��
(3) ��������Һʱ��������������ķ�����___________________________��
(4) д������������Ӧ�����ӷ���ʽ��___________________________________��
(5) ��μ���Ni(OH)3��ϴ�Ӹɾ���_______________________��
����Ŀ������ʵ������淶���ܴﵽʵ��Ŀ�ĵ���
���� | Ŀ�� | |
A | ��ȡ5.0gCuSO4��5H2O����27.0gˮ��,�����ܽ� | ����10%CuSO4��Һ |
B | ����ϡ����ϴ�ӣ�����ˮ��ϴ | ϴ�ӷֽ�KMnO4��O2���Թ� |
C | �ò�����պȡ��Һ�����ڸ����pH��ֽ�ϣ�Ƭ�̺������ɫ���Ƚϲ����� | �ⶨ0.05mol.L-1NaClO��Һ��pH |
D | ���ֵ�����ձ��У��ձ��ڷ�һʢ����ˮ����ƿ����ʯ�������ձ����ȣ�Ȼ���ռ���ƿ��ڵĹ��� | �ᴿ����NH4Cl�Ĵֵ� |
A. A B. B C. C D. D
����Ŀ��ijС��ͬѧ��FeCl3��KI�ķ�Ӧ����̽����
������̽���������½����±�����ʵ�顣
��� | ���� | ���� |
ʵ���� | ȡ5mL 0.1mol/L KI��Һ���μ�0.1mol/L FeCl3��Һ56�Σ������ҺpH��5�� | ��Һ��Ϊ�ػ�ɫ |
ʵ���� | ȡ2mLʵ������Ӧ�����Һ���μ�2��0.1molL1 KSCN��Һ | ��Һ�ʺ�ɫ |
(1)֤��ʵ��������Fe2+ ���ɣ�������Լ�Ϊ____________________________��
(2)д��ʵ������Ӧ�����ӷ���ʽ��_____________________________________��
(3)����ʵ���������֤��Fe3+��I�������淴Ӧ��ʵ����������ϵ�������__________
(4)��ʵ��I����Һ�м���CCl4��ʵ��������____________________________��ȡ���ϲ���Һ�еμ�KSCN��Һ����δ�������Եĺ�ɫ����ԭ��Ϊ����ƽ���ƶ��ĽǶȽ��ͣ�_________________________________________________________��
������̽����20min������۲�ʵ������ʵ������Һ�ػ�ɫ���ʵ������Һ��ɫ��dz��
(5)��֪�����Խ�ǿ�������£�I�ɱ���������ΪI2���ʼ�ͬѧ������裺�÷�Ӧ�����¿�����I����ΪI2��ʹʵ�������Һ�ػ�ɫ�����ͬѧ���ʵ�飺____________________________________��20min����Һ��������֤���ü��費������������Һ�����������ؿ�����__________________________��